Chemistry:Organotechnetium compound
Organotechnetium compounds in organometallic chemistry contain carbon-to-technetium chemical bonds. Organotechnetium chemistry is the science of organotechnetium compounds describing their physical properties, synthesis and reactions. They are most widely used as a radiopharmaceutical imaging agent.[1] Most commonly as Coordination complexes, although organotechnetium radiopharmaceuticals are emerging.[2] Organotechnetium compounds are not typically used in Chemical reactions or catalysis due to their radioactivity. Research on technetium chemistry is often done in conjunction with rhenium as a isoelectronic non-radioactive alternative to technetium.[3]
Brief history
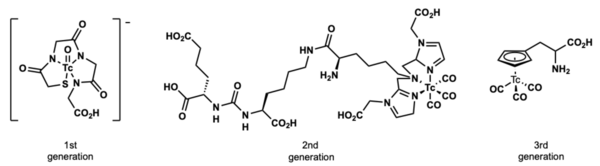
Technetium were first used as a radiopharmaceutical in 1961.[4] Of the radiopharmaceuticals in clinical use for SPECT (single photon emission computed tomography), a majority of the compounds are 99mTc complexes.[5] Three generations of technetium radiopharmaceuticals currently exist and are used. The first generation do not localize specifically and are considered perfusion agents. Second generation has a peptide based targeting portion. The third generation of technetium radiopharmaceuticals features organotechnetium compounds that can localize in the body in a biomimetic manner.[6][7][8]
Examples
A vast majority of technetium compounds used in radiopharmaceutical imaging and diagnosis are inorganic coordination complexes. There are a number of “classical” organometallic organotechnetium compounds, specifically containing carbon-technetium bonds are in clinical use. These organotechnetium compounds are mostly seen as technetium tri-carbonyl compounds and technetium cyclopentadienyl compounds.
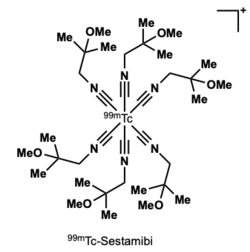
One of the most prominent radio pharmaceutical compounds in clinical use is Cardiolie®, also known as 99mTc-Sestamibi. This organotechnetium compound is applied for myocardial imaging. The d6 electron configuration is highly stable due to its low oxidation state.[9] The Tc(I) complex is further stabilized by the high reducing potential of the isonitrile ligands.[10]
The above piano-stool organotechnetium complex is a third generation radiopharmaceutical. The cyclopentadienyl ligand acts as a bio isostere to a phenyl group in the amino acid phenylalanine.[11]
Synthesis
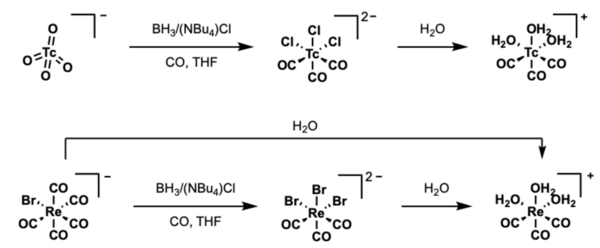
Radioactive 99mTc is obtained in the pertechnetate form in dilute aqueous solution from 99Mo/99mTc generators. Pertechnetate can then be made into more useful carbonyl and hydrate precursors for subsequent synthesis into technetate complexes.
As the starting radiometals are most available in aqueous solution due to method of isolation, the chemistry for synthesis of technetate compounds must be done in aqueous solution.
The study of technetium compounds is typically done in conjugation with rhenium as an isoelectronic and non-radioactive alternative to technetium.
Precursors
For 99mTc and 188Re, the synthesis of compounds start with pertechnetate or perrhenate in saline at low concentration, obtained from 99Mo/99mTc and 188W/188Re generators. The aquo tricarbonyl precursors are useful for accessing Tc and Re complexes. The metals have d6 low-spin electronic configuration, providing high kinetic stability, and highly stable M-C bonds. Consequently, the three CO ligands always remain coordinated, while ligands readily replace the three water molecules. Typical organotechnetium compounds thus feature the tricarbonyl motif.
Typical methods of organometallic compounds synthesis difficult to utilize. To be useful as a radiopharmaceutical, the reaction should be done in an aqueous saline solution that can be injected into the body intravenously.
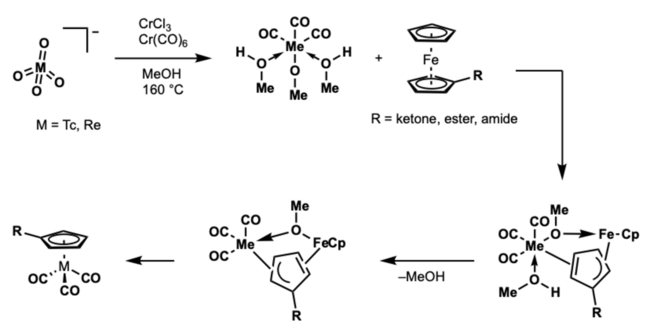
Double Ligand Transfer
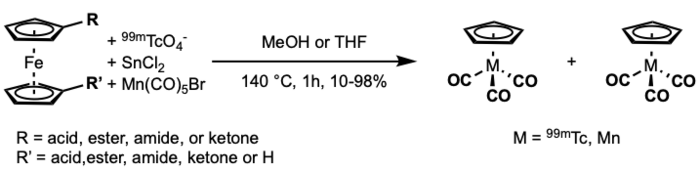
A double ligand transfer (DLT) reaction was developed by Martin Wenzel for synthesis of organotechnetium/organorhenium complexes.[12] The reaction features the synthesis of organotechnetium piano-stool compounds from ferrocene. The reaction was further studied and optimized by Katzenellegbogen.[13] Unfortunately, the utility of this method in the synthesis of radiopharmaceuticals is limited by the use of organic solvent.
Mechanism
This mechanism is proposed to proceed by ring slippage. First, reduction and carbonylation of the pertechnetate/perrhenate with CrCl3 and/or Cr(CO)6 to from the 6 coordinate intermediate. Subsequent reaction with the substituted ferrocene through ring-slipped, bridged intermediates then gives product. The transfer of the more electron deficient ring is favored by the stabilization of the transition state of η5- η3 ring slip of ferrocene.
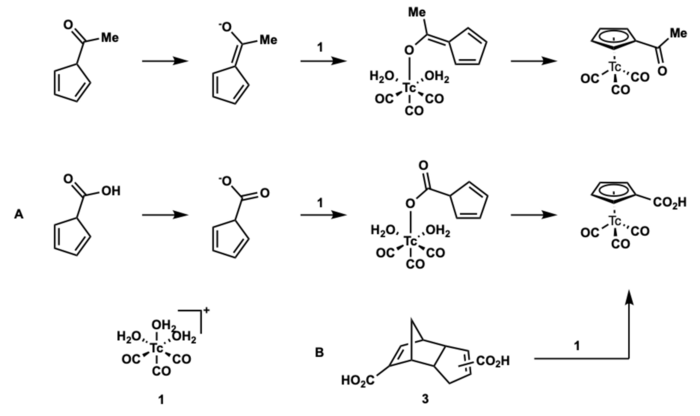
Metal-Mediated Retro Diels-Alder
Aqueous synthesis enables development for medically relevant radiopharmaceuticals. First aqueous synthesis of fac-[99mTc(η5 -Cp-C(O)CH3)(CO)3] was described by the Alberto lab utilized a metal-mediated retro Diels-Alder to synthesize the organotechnetium complexes.[14]
Mechanism
In a step-wise manner, the carboxylate first coordinates to technetium followed by coordination to the adjacent cyclopentadiene (Path A). The reaction is thermodynamically driven, given a strong electronic interaction between [99mTc(CO)3]+ and the cyclopentadiene.
The favorable formation of the {(η5-Cp)Tc} as a driving force for formation of the product 2, prompted the use of the Diels-Ader dimer (HCp-COOH)2 (Thiele’s acid) as a precursor to the cyclopentadiene. Thermal cracking of 3 typically requires T >160 °C. Reaction of 3 and 1 at 95 °C for 30 min in buffer gave quantitative formation of 2. As no free HCp-COOH was observed, in situ retro Diels-Alder and subsequent entry into path A was excluded.
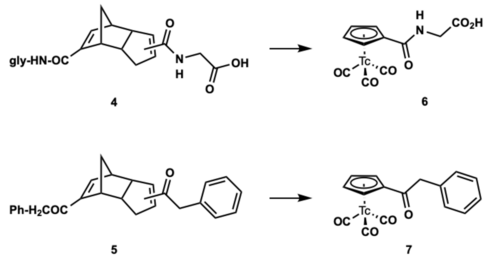
Examples
The metal-mediated retro Diels-Alder reaction suggests a general approach to [(Cp-R)99mTc(CO)3], enable access to a variety of R groups on the Cp ring.
With the development of this retro Diels-Alder method for synthesis of 99mTc and Re complexes in aqueous media by the Alberto lab, The labeling of biomolecules with piano-stool like complexes is now possible. Enabling access to the development of novel radiopharmaceuticals.
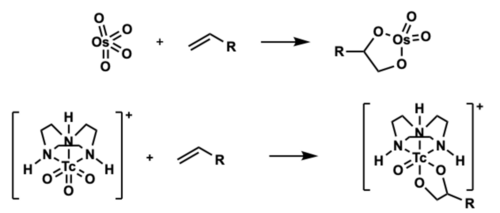
Reactivity
Technetium has been shown to react similarity to osmium. Able to catalyze a cis dihydroxylation.
References
- ↑ "Technetium Radiopharmaceutical Chemistry.". The University of New Mexico. Health Sciences Center, Colloge of Pharmacy. 2006. pp. 1–77. https://pharmacyce.unm.edu/nuclear_program/freelessonfiles/vol12lesson3.pdf.
- ↑ "Good reasons to study technetium chemistry". Chemical & Engineering News 95 (15). April 2017. https://cen.acs.org/articles/95/i15/reasons-study-technetium-chemistry.html. Retrieved 2021-06-12.
- ↑ Jürgens, Sophie; Herrmann, Wolfgang A.; Kühn, Fritz E. (February 2014). "Rhenium and technetium based radiopharmaceuticals: Development and recent advances" (in en). Journal of Organometallic Chemistry 751: 83–89. doi:10.1016/j.jorganchem.2013.07.042. https://linkinghub.elsevier.com/retrieve/pii/S0022328X13005494.
- ↑ Scerri, Eric (July 2009). "Tales of technetium" (in en). Nature Chemistry 1 (4): 332. doi:10.1038/nchem.271. ISSN 1755-4330. PMID 21378873. Bibcode: 2009NatCh...1..332S. http://www.nature.com/articles/nchem.271.
- ↑ Morais, Goreti Ribeiro; Paulo, António; Santos, Isabel (2012-08-27). "Organometallic Complexes for SPECT Imaging and/or Radionuclide Therapy" (in en). Organometallics 31 (16): 5693–5714. doi:10.1021/om300501d. ISSN 0276-7333. https://pubs.acs.org/doi/10.1021/om300501d.
- ↑ Schibli, Roger; Schubiger, August (2002-11-01). "Current use and future potential of organometallic radiopharmaceuticals". European Journal of Nuclear Medicine and Molecular Imaging 29 (11): 1529–1542. doi:10.1007/s00259-002-0900-8. ISSN 1619-7070. PMID 12397472. http://link.springer.com/10.1007/s00259-002-0900-8.
- ↑ Liu, Shuang (2004-09-08). "The role of coordination chemistry in the development of target-specific radiopharmaceuticals" (in en). Chemical Society Reviews 33 (7): 445–461. doi:10.1039/B309961J. ISSN 1460-4744. PMID 15354226. https://pubs.rsc.org/en/content/articlelanding/2004/cs/b309961j.
- ↑ Hirao, Toshikazu (2018). Advances in Bioorganometallic Chemistry. Toshiyuki Moriuchi. San Diego: Elsevier. ISBN 978-0-12-814198-4. OCLC 1079003267. https://www.worldcat.org/oclc/1079003267.
- ↑ Alberto, Roger; Herrmann, Wolfgang A.; Bryan, Jeff C.; Schubiger, P. August; Baumgartner, Franz; Mihalios, Dimitrios (1993-01-01). "New Organometallic Technetium Complexes in High and Low Oxidation States". Radiochimica Acta 63 (s1): 153–162. doi:10.1524/ract.1993.63.special-issue.153. ISSN 2193-3405. https://www.degruyter.com/document/doi/10.1524/ract.1993.63.special-issue.153/html.
- ↑ Claude, Guilhem; Salsi, Federico; Hagenbach, Adelheid; Gembicky, Milan; Neville, Michael; Chan, Chinglin; Figueroa, Joshua S.; Abram, Ulrich (2020-06-22). "Structural and Redox Variations in Technetium Complexes Supported by m -Terphenyl Isocyanides" (in en). Organometallics 39 (12): 2287–2294. doi:10.1021/acs.organomet.0c00238. ISSN 0276-7333. https://pubs.acs.org/doi/10.1021/acs.organomet.0c00238.
- ↑ Johannsen, Bernd; Syhre, Rosemarie; Spies, Hartmut; Münze, Rudolf (1978-07-01). "Chemical and Biological Characterization of Different Tc Complexes of Cysteine and Cysteine Derivatives" (in en). Journal of Nuclear Medicine 19 (7): 816–824. ISSN 0161-5505. PMID 660286. https://jnm.snmjournals.org/content/19/7/816.
- ↑ Spradau, Todd W.; Katzenellenbogen, John A. (1998-05-01). "Preparation of Cyclopentadienyltricarbonylrhenium Complexes Using a Double Ligand-Transfer Reaction". Organometallics 17 (10): 2009–2017. doi:10.1021/om971018u. ISSN 0276-7333. https://doi.org/10.1021/om971018u.
- ↑ "John A. Katzenellenbogen | Chemistry at Illinois" (in en). https://chemistry.illinois.edu/jkatzene.
- ↑ Liu, Yu; Spingler, Bernhard; Schmutz, Paul; Alberto, Roger (2008-02-06). "Metal-Mediated Retro Diels−Alder of Dicyclopentadiene Derivatives: A Convenient Synthesis of [(Cp-R)M(CO)3 (M = 99mTc, Re) Complexes"]. Journal of the American Chemical Society 130 (5): 1554–1555. doi:10.1021/ja077741l. ISSN 0002-7863. PMID 18186638. https://doi.org/10.1021/ja077741l.