Chemistry:Pertechnetate
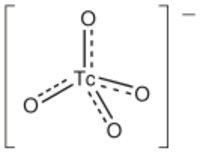
The pertechnetate ion (/pərˈtɛknəteɪt/)[1] is an oxyanion with the chemical formula TcO−4. It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the 99mTc isotope (half-life 6 hours) which is commonly used in nuclear medicine in several nuclear scanning procedures.
A technetate(VII) salt is a compound containing this ion. Pertechnetate compounds are salts of technetic(VII) acid. Pertechnetate is analogous to permanganate but it has little oxidizing power. Pertechnetate has higher oxidation power than perrhenate.[2]
Understanding pertechnetate is important in understanding technetium contamination in the environment and in nuclear waste management.[2]
Chemistry
TcO−4 is the starting material for most of the chemistry of technetium. Pertechnetate salts are usually colorless.[3] TcO−4 is produced by oxidizing technetium with nitric acid or with hydrogen peroxide. The pertechnetate anion is similar to the permanganate anion but is a weaker oxidizing agent. It is tetrahedral and diamagnetic. The standard electrode potential for TcO−4/TcO2 is only +0.738 V in acidic solution, as compared to +1.695 V for MnO−4/MnO2.[4] Because of its diminished oxidizing power, TcO−4 is stable in alkaline solution. TcO−4 is more similar to ReO−4. Depending on the reducing agent, TcO−4 can be converted to derivatives containing Tc(VI), Tc(V), and Tc(IV).[5] In the absence of strong complexing ligands, TcO−4 is reduced to a +4 oxidation state via the formation of TcO2 hydrate.[4]
Preparation of 99mTcO4−
99mTc is conveniently available in high radionuclidic purity from molybdenum-99, which decays with 87% probability to 99mTc. The subsequent decay of 99mTc leads to either 99Tc or 99Ru. 99Mo can be produced in a nuclear reactor via irradiation of either molybdenum-98 or naturally occurring molybdenum with thermal neutrons, but this is not the method currently in use today. Currently, 99Mo is recovered as a product of the nuclear fission reaction of 235U,[6] separated from other fission products via a multistep process and loaded onto a column of alumina that forms the core of a 99Mo/99mTc radioisotope generator.
As the 99Mo continuously decays to 99mTc, the 99mTc can be removed periodically (usually daily) by flushing a saline solution (0.15 M NaCl in water) through the alumina column: the more highly charged 99MoO2−4 is retained on the column, where it continues to undergo radioactive decay, while the medically useful radioisotope 99mTcO−4 is eluted in the saline. The eluate from the column must be sterile and pyrogen free, so that the Tc drug can be used directly, usually within 12 hours of elution.[4] In a few cases, sublimation or solvent extraction may be used.
Examples
- A complex that can penetrate the blood–brain barrier is generated by reduction of 99mTcO−4 with tin(II) in the presence of the ligand hexamethylpropylene amine oxime (HMPAO) to form TcO-D,L-HMPAO.
- A complex that for imaging the lungs, Tc-MAA, is generated by reduction of 99mTcO−4 with SnCl2 in the presence of human serum albumin.
- [99mTc(OH2)3(CO)3]+, which is both water and air stable, is generated by reduction of 99mTcO−4 with carbon monoxide. This compound is a precursor to complexes that can be used in cancer diagnosis and therapy involving DNA-DNA pretargeting.[7]
Compounds
| Formula | name | crystal structure | cell dimensions (Å) | unit cell volume (Å3) | remarks | references |
|---|---|---|---|---|---|---|
| LiTcO4 | lithium pertechnetate | [2] | ||||
| LiTcO4·2H2O | lithium pertechnetate dihydrate | [2] | ||||
| LiTcO4·3H2O | lithium pertechnetate trihydrate | Pt3/mc | [2] | |||
| NaTcO4 | sodium pertechnetate | tetragonal | a = 5.342, c = 1.874 | 338.91 | absorbs water from atmosphere | [2] |
| NaTcO4·H2O | sodium pertechnetate monohydrate | [2] | ||||
| NaTcO4·2H2O | sodium pertechnetate dihydrate | [2] | ||||
| NaTcO4·4H2O | sodium pertechnetate tetrahydrate | [2] | ||||
| KTcO4 | potassium pertechnetate | tetragonal | a = 5.647, c = 12.91 | 411.73 | used to prepare radiopharmaceuticals | [2] |
| RbTcO4 | rubidium pertechnetate | tetragonal | a = 5.762, c =13.543 | 449.65 | [2] | |
| α-CsTcO4 | α-caesium pertechnetate | tetragonal | a = 5.898, c = 14.38 | volatile at temperatures >470K | [2] | |
| β-CsTcO4 | β-caesium pertechnetate | orthorhombic | a = 5.737, b = 5.92, c = 14.341 | 486.38 | [2] | |
| TlTcO4 | thallium pertechnetate | orthorhombic | [2] | |||
| TlTcO4 | thallium pertechnetate | tetragonal | [2] | |||
| NH4TcO4 | ammonium pertechnetate | tetragonal | technetium may be supplied in this form | [2] | ||
| AgTcO4 | silver pertechnetate | tetragonal | [2] |
Reactions
- Radiolysis of TcO−4 in nitrate solutions proceeds through the reduction to TcO2−4 which induces complex disproportionation processes:
- Pertechnetate can be reduced by H2S to give Tc2S7.[8]
- Pertechnetate is also reduced to Tc(IV/V) compounds in alkaline solutions in nuclear waste tanks without adding catalytic metals, reducing agents, or external radiation. Reactions of mono- and disaccharides with 99mTcO−4 yield Tc(IV) compounds that are water-soluble.[9]
Uses
Pharmaceutical use
The half-life of 99mTc is long enough that labelling synthesis of the radiopharmaceutical and scintigraphic measurements can be performed without significant loss of radioactivity.[4] The energy emitted from 99mTc is 140 keV, which allows for the study of deep body organs. Radiopharmaceuticals have no intended pharmacologic effect and are used in very low concentrations. Radiopharmaceuticals containing 99mTc are currently being applied in the determining morphology of organs, testing of organ function, and scintigraphic and emission tomographic imaging. The gamma radiation emitted by the radionuclide allows organs to be imaged in vivo tomographically. Currently, over 80% of radiopharmaceuticals used clinically are labelled with 99mTc. A majority of radiopharmaceuticals labelled with 99mTc are synthesized by the reduction of the pertechnetate ion in the presence of ligands chosen to confer organ specificity of the drug. The resulting 99mTc compound is then injected into the body and a "gamma camera" is focused on sections or planes in order to image the spatial distribution of the 99mTc.
Specific imaging applications
99mTc is used primarily in the study of the thyroid gland - its morphology, vascularity, and function. TcO−4 and iodide, due to their comparable charge/radius ratio, are similarly incorporated into the thyroid gland. The pertechnetate ion is not incorporated into the thyroglobulin. It is also used in the study of blood perfusion, regional accumulation, and cerebral lesions in the brain, as it accumulates primarily in the choroid plexus.
Pertechnetate salts, such as sodium pertechnetate, cannot pass through the blood–brain barrier. In addition to the salivary and thyroid glands, 99mTcO−4 localizes in the stomach. 99mTcO−4 is renally eliminated for the first three days after being injected. After a scanning is performed, it is recommended that a patient drink large amounts of water in order to expedite elimination of the radionuclide.[10] Other methods of 99mTcO−4 administration include intraperitoneal, intramuscular, subcutaneous, as well as orally. The behavior of the 99mTcO−4 ion is essentially the same, with small differences due to the difference in rate of absorption, regardless of the method of administration.[11]
Synthesis of 99mTcO4− radiopharmaceuticals
99mTcO−4 is advantageous for the synthesis of a variety of radiopharmaceuticals because Tc can adopt a number of oxidation states.[4] The oxidation state and coligands dictate the specificity of the radiopharmaceutical. The starting material Na[99mTcO4], made available after elution from the generator column, as mentioned above, can be reduced in the presence of complexing ligands. Many different reducing agents can be used, but transition metal reductants are avoided because they compete with 99mTc for ligands. Oxalates, formates, hydroxylamine, and hydrazine are also avoided because they form complexes with the technetium. Electrochemical reduction is impractical.
Ideally, the synthesis of the desired radiopharmaceutical from 99mTcO−4, a reducing agent, and desired ligands should occur in one container after elution, and the reaction must be performed in a solvent that can be injected intravenously, such as a saline solution. Kits are available that contain the reducing agent, usually tin(II) and ligands. These kits are sterile, pyrogen-free, easily purchased, and can be stored for long periods of time. The reaction with 99mTcO−4 takes place directly after elution from the generator column and shortly before its intended use. A high organ specificity is important because the injected activity should accumulate in the organ under investigation, as there should be a high activity ratio of the target organ to nontarget organs. If there is a high activity in organs adjacent to the one under investigation, the image of the target organ can be obscured. Also, high organ specificity allows for the reduction of the injected activity, and thus the exposure to radiation, in the patient. The radiopharmaceutical must be kinetically inert, in that it must not change chemically in vivo en route to the target organ.
As a 99mTc carrier
A technetium-99m generator provides the pertechnetate containing the short-lived isotope 99mTc for medical uses. This compound is generated directly from molybdate held on alumina within the generator (see this topic for detail).
In nuclear medicine
Pertechnetate has a wide variety of uses in diagnostic nuclear medicine. Since technetate(VII) can substitute for iodine in the Na/I symporter (NIS) channel in follicular cells of the thyroid gland, inhibiting uptake of iodine into the follicular cells, 99mTc-pertechnetate can be used as an alternative to 123I in imaging of the thyroid, although it specifically measures uptake and not organification.[12] It has also been used historically to evaluate for testicular torsion, although ultrasound is more commonly used in current practice, as it does not deliver a radiation dose to the testes. It is also used in labeling of autologus red blood cells for MUGA scans to evaluate left ventricular cardiac function, localization of gastrointestinal bleeding prior to embolization or surgical management, and in damaged red blood cells to detect ectopic splenic tissue.
It is actively accumulated and secreted by the mucoid cells of the gastric mucosa,[13] and therefore, technetate(VII) radiolabeled with technetium-99m is injected into the body when looking for ectopic gastric tissue as is found in a Meckel's diverticulum with Meckel's scans.[14]
Non-radioactive uses
All technetium salts are mildly radioactive, but some of them have explored use of the element for its chemical properties. In these uses, its radioactivity is incidental, and generally the least radioactive (longest-lived) isotopes of Tc are used. In particular, 99Tc (half-life 211,000 years) is used in corrosion research, because it is the decay product of the easily obtained commercial 99mTc isotope.[2] Solutions of technetate(VII) react with the surface of iron to form technetium dioxide, in this way it is able to act as an anodic corrosion inhibitor.[15]
See also
References
- ↑ "Pertechnetate". Merriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/pertechnetate.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 Weaver, Jamie; Soderquist, Chuck Z.; Washton, Nancy M.; Lipton, Andrew S.; Gassman, Paul L.; Lukens, Wayne W.; Kruger, Albert A.; Wall, Nathalie A. et al. (21 February 2017). "Chemical Trends in Solid Alkali Pertechnetates". Inorganic Chemistry 56 (5): 2533–2544. doi:10.1021/acs.inorgchem.6b02694. PMID 28221786.
- ↑ Wells, A. F.; Structural Inorganic Chemistry; Clarendon Press: Oxford, Great Britain; 1984; p. 1050.
- ↑ 4.0 4.1 4.2 4.3 4.4 Schwochau, K. (1994). "Technetium Radiopharmaceuticals-Fundamentals, Synthesis, Structure, and Development". Angew. Chem. Int. Ed. Engl. 33 (22): 2258–2267. doi:10.1002/anie.199422581.
- ↑ Encyclopædia Britannica: Technetium
- ↑ Beasley, T. M., Palmer, H. E., and Nelp, W. B. (1966). "Distribution and Excretion of Technetium in Humans". Health Physics 12 (10): 1425–1435. doi:10.1097/00004032-196610000-00004. PMID 5972440. http://www.health-physics.com/pt/re/healthphys/abstract.00004032-196610000-00004.htm.
- ↑ R. Alberto, R. Schibli, A. Egli, A. P. Schubiger, U. Abram and T. A. Kaden (1998). "A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]− in Aqueous Solution and Its Reaction with a Bifunctional Ligand". J. Am. Chem. Soc. 120 (31): 7987–7988. doi:10.1021/ja980745t.
- ↑ Emeléus, H. J.; Sharpe, A. G. (1968). Advances in Inorganic Chemistry and Radiochemistry, Volume 11. Academic Press. pp. 26. ISBN 978-0-08-057860-6. https://books.google.com/books?id=-SnCsg5jM_kC&pg=PA26.
- ↑ D. E. Berning, N. C. Schroeder and R. M. Chamberlin (2005). "The autoreduction of pertechnetate in aqueous, alkaline solutions". Journal of Radioanalytical and Nuclear Chemistry 263 (3): 613–618. doi:10.1007/s10967-005-0632-x. https://zenodo.org/record/1232814.
- ↑ Shukla, S. K., Manni, G. B., and Cipriani, C. (1977). "The Behaviour of the Pertechnetate Ion in Humans". Journal of Chromatography B 143 (5): 522–526. doi:10.1016/S0378-4347(00)81799-5. PMID 893641.
- ↑ Razzak, M. A.; Naguib, M.; El-Garhy, M. (1967). "Fate of Sodium Pertechnetate-Technetium-99m". Journal of Nuclear Medicine 8 (1): 50–59. PMID 6019138.
- ↑ Ryo, U.Y.; Vaidya, P.V.; Schneider, A.B.; Bekerman, C; Pinsky, S.M. (1983). "Thyroid imaging agents: a comparison of I-123 and Tc-99m pertechnetate". Radiology 148 (3): 819–822. doi:10.1148/radiology.148.3.6308711. PMID 6308711.
- ↑ Nuclear Imaging of Meckel's Diverticulum: A Pictorial Essay of Pitfalls S. Huynh, M.D., R. Amin, M.D., B. Barron, M.D., R. Dhekne, M.D., P. Nikolaidis, M.D., L. Lamki, M.D.. University of Texas Houston Medical School and Memorial Hermann - Texas Medical Center (TMC), St. Luke's Episcopal Hospital and Texas Children Hospital, Houston, Texas. Last Modified September 5, 2007
- ↑ Diamond, Robert; Rothstein, Robin; Alavi, Abass (1991). "The Role of Cimetidine-Enhanced Technetium 99m-Pertechnetate Imaging for Visualizing Meckel's Diverticulum". The Journal of Nuclear Medicine 32 (7): 1422–1424. PMID 1648609. http://jnm.snmjournals.org/cgi/reprint/32/7/1422.pdf.
- ↑ Cartledge, G. H. (1973). "Twenty-Year Inhibition of Corrosion by the Pertechnetate Ion". Corrosion 29 (9): 361–362. doi:10.5006/0010-9312-29.9.361.
 |
