Chemistry:Paclobutrazol
| |

| |
| Names | |
|---|---|
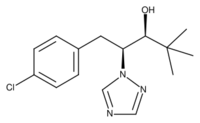
| IUPAC name
(2RS,3RS)-1-(4-Chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)-3-pentanol
| |
| Other names
PP333
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| Abbreviations | PBZ |
| ChEBI |
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties[1] | |
| C15H20ClN3O | |
| Molar mass | 293.80 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.23 g/cm3 |
| Melting point | 165-166°C |
| Boiling point | 460.9 °C (861.6 °F; 734.0 K) at 760 mHg |
| 22.9 mg/L (20 °C) | |
| log P | 3.11 |
| Hazards | |
| Main hazards | Xn |
| Flash point | 232.6 °C (450.7 °F; 505.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Paclobutrazol (PBZ) is the ISO common name for an organic compound that is used as a plant growth retardant and triazole fungicide.[2][3] It is a known antagonist of the plant hormone gibberellin, acting by inhibiting gibberellin biosynthesis, reducing internodal growth to give stouter stems, increasing root growth, causing early fruitset and increasing seedset in plants such as tomato and pepper. PBZ has also been shown to reduce frost sensitivity in plants. Moreover, paclobutrazol can be used as a chemical approach for reducing the risk of lodging in cereal crops. PBZ has been used by arborists to reduce shoot growth and shown to have additional positive effects on trees and shrubs. Among those are improved resistance to drought stress, darker green leaves, higher resistance against fungi and bacteria, and enhanced development of roots. Cambial growth, as well as shoot growth, has been shown to be reduced in some tree species.
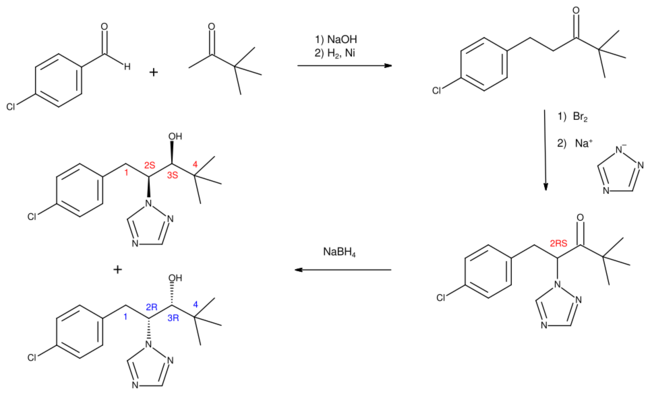
Structure and synthesis
The first synthesis of paclobutrazol was disclosed in patents filed by an ICI group working at Jealott's Hill.[4]
4-Chlorobenzaldehyde and pinacolone are combined in an aldol condensation to form a chalcone which is hydrogenated using Raney nickel as catalyst to give a substituted ketone. This material is brominated and the resulting compound treated with the sodium salt of 1,2,4-triazole in a nucleophilic substitution reaction. The final reduction reaction uses sodium borohydride, which in cold methanol gives almost exclusively the diastereomer pair having the absolute configuration (2R,3R) and its enantiomer (2S,3S), with only about 2% of the alternative (2R,3S) and (2S,3R) isomers. However, this pair of isomers can be produced when the reduction is carried out using butylmagnesium bromide.[5]
In a 1984 study, ICI workers separated the individual enantiomers by chiral resolution and were able to demonstrate that only the (2R,3R) isomer displays substantial fungicidal activity, whereas the (2S,3S) isomer is responsible for the growth regulating properties.[5][6] However, the commercial product (developed under the code number PP333) was the racemic material, since separation of the isomers was unnecessary when both components had utility in agriculture.[2][7]
Mechanism of action
Paclobutrazol is an inhibitor of enzymes which use cytochrome P450 as a co-factor. Their active site contains a heme center which activates oxygen from the air to oxidise their substrates. The (2S,3S) isomer inhibits the enzyme ent-kaurene oxidase[5][8] which is on the main biosynthetic pathway to gibberellins, which are important plant hormones.[9][10] A secondary effect arising from the inhibition of ent-kaurene oxidase is that its precursor, geranylgeranyl pyrophosphate accumulates in the plant and some of this is diverted into additional production of the phytol group of chlorophyll and the hormone abscisic acid. The latter is responsible for controlling transpiration of water through the leaves and hence PBZ treatment can lead to better tolerance of drought conditions.[11] The (2R,3R) isomer is a better fit to the active site of the fungal cytochrome P450 14α-demethylase. This inhibits the conversion of lanosterol to ergosterol, a component of the fungal cell membrane, which is lethal for many species.[5] Many other azole derivatives including propiconazole and tebuconazole show this type of activity, so the main commercial opportunity for paclobutrazol was as a plant growth retardant and it was first marketed by ICI in 1985 under the trade names Bonzi, Clipper, Cultar and Parlay.[1][7]
Usage
As an antagonist of gibberellin biosynthesis, PBZ has a growth retardant effect on most plant species.[11] It is absorbed by plant tissues and transported via the xylem to the growing parts, where the rate of cell division is reduced compared to untreated plants and the new cells do not elongate.[7][12]:5
Ornamental crops
PBZ is used in horticulture, especially for glasshouse-reared perennial plants.[13][14]
Trees and shrubs
The ability of PBZ to reduce the growth of trees and shrubs means that it has found use in areas where there is a need to moderate such growth, for example under electric power lines and where a right-of-way is to be maintained. A single application of the growth regulator can give season-long control.[12][15]
Fruit and vegetables
PBZ is used to increase the quantity and quality of orchard fruit and of vegetables. The quality is measured by elevated amounts of carbohydrates, total soluble solids (TSS), the TSS/titratable acidity ratio and a decreased acidity.[11] It stimulates the growth of roots and stems and maintains the number of the leaves but suppresses the height of the plants.[16][17][18]
Turf management
PBZ has been extensively used as a means to improve the quality of turf on golf courses, where it reduces the need for mowing and by increasing chlorophyll content has the effect of greening the grass.[19][20]
Cereal crops
By diverting the plant's productivity from stem elongation into seed production, PBZ is demonstrated to increase grain yields and reduce lodging, demonstrated by Kamran et al., 2017[21][3][11] and Tekalign 2007.[22][3][11] The same mechanism is responsible for modern high-yield semi-dwarf crops such as the IR8 rice variety.[23] Peng et al., 2014 also describe better lodging tolerance.[24] They find that winter wheat undergoes reduction of internode length, thickened internodes, increased lateral growth, increased lignin synthesis enzyme activity and therefore increased lignification with application of this compound.[24] Although this does not reduce lodging it does make lodging less harmful.[24]
Effects on the environment
PBZ has been the subject of extensive regulatory studies, including in the European Union[25] and the US.[12] These data have been summarised.[1] It was assessed as being of moderate acute toxicity, mildly irritating to skin and eyes and unlikely to be genotoxic or carcinogenic to humans.[25] PBZ is relatively stable in water and soil. Under laboratory aerobic or anaerobic conditions, the half-life of paclobutrazol can be higher than one year.[25] However, in a 2010 quantitative analysis, PBZ was detected in only 3 out of 440 groundwater samples from golf turf areas with a maximum concentration of 4.2 µg/L.[20] In Europe, the highest tolerable concentration of paclobutrazol in drinking water is 66 µg/L.[12]
As research tool
PBZ has been used as a tool to investigate the genes associated with gibberellin biosynthesis in plants. For example, the Arabidopsis allele gai-t6 (of the giberellic acid interacting gene) confers resistance to paclobutrazol's damage to vegetative growth.[26][27] However, in normal use, there is no selective pressure on plants to develop resistance to PBZ since it is not lethal to them.
References
- ↑ 1.0 1.1 1.2 Pesticide Properties Database. "Paclobutrazol". University of Hertfordshire. http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/504.htm.
- ↑ 2.0 2.1 "Compendium of Pesticide Common Names: paclobutrazol". BCPC. https://pesticidecompendium.bcpc.org/paclobutrazol.html.
- ↑ 3.0 3.1 3.2 Tesfahun, Wakjira (January 1, 2018). Yildiz, Fatih. ed. "A review on: Response of crops to paclobutrazol application". Cogent Food & Agriculture 4 (1): 1–9. doi:10.1080/23311932.2018.1525169.
- ↑ ; Sugavanam, B. & Shephard, M.C. et al."Triazoles and imidazoles useful as plant fungicides and growth regulating agents" GB patent 1595696, issued 1981-08-12, assigned to ICI Ltd
- ↑ 5.0 5.1 5.2 5.3 Sugavanam, Balasubramanyan (1984). "Diastereoisomers and enantiomers of paclobutrazol: Their preparation and biological activity". Pesticide Science 15 (3): 296–302. doi:10.1002/ps.2780150312.
- ↑ Black, S.N.; Williams, L.J.; Davey, R.J.; Moffatt, F.; Jones, R.V.H.; McEwan, D.M.; Sadler, D.E. (1989). "The preparation of enantiomers of paclobutrazol: A crystal chemistry approach". Tetrahedron 45 (9): 2677–2682. doi:10.1016/S0040-4020(01)80097-1.
- ↑ 7.0 7.1 7.2 Worthing C.R., ed (1987). The Pesticide Manual - A World Compendium (Eighth ed.). British Crop Protection Council. pp. 628–629. ISBN 0948404019.
- ↑ Davidson, Sandra E.; Smith, Jennifer J.; Helliwell, Chris A.; Poole, Andrew T.; Reid, James B. (2004). "The Pea Gene LH Encodes ent-Kaurene Oxidase". Plant Physiology 134 (3): 1123–1134. doi:10.1104/pp.103.032706. PMID 14988475.
- ↑ Rademacher, Wilhelm (June 2000). "Growth Retardants: Effects on Gibberellin Biosynthesis and Other Metabolic Pathways". Annual Review of Plant Physiology and Plant Molecular Biology 51 (1): 501–531. doi:10.1146/annurev.arplant.51.1.501. PMID 15012200.
- ↑ Hedden, Peter (2020). "The Current Status of Research on Gibberellin Biosynthesis". Plant and Cell Physiology 61 (11): 1832–1849. doi:10.1093/pcp/pcaa092. PMID 32652020.
- ↑ 11.0 11.1 11.2 11.3 11.4 Desta, Bizuayehu; Amare, Getachew (January 7, 2021). "Paclobutrazol as a plant growth regulator". Chemical and Biological Technologies in Agriculture 8 (1). doi:10.1186/s40538-020-00199-z.
- ↑ 12.0 12.1 12.2 12.3 "Active Ingredient Paclobutrazol: Review Conducted by MDAR and MassDEP for Use in Sensitive Areas of Rights-of-Way in Massachusetts.". January 2012. https://www.mass.gov/doc/paclobutrazol-review-jan-2012pdf/download?_ga=2.169004968.820722649.1646429016-1603828005.1646429016. Retrieved 4 February 2022.
- ↑ Whipker, Brian E. (2022). "Wide Assortment of Available PGRs". GrowerTalks (Ball Publishing): 10–12. https://www.fine-americas.com/wp-content/uploads/2022/01/PGR_Guide_2022-2023.pdf. Retrieved 2022-04-06.
- ↑ Latimer, Joyce G. (2022). "Growth Regulators for Containerized Herbaceous Perennial Plants". GrowerTalks (Ball Publishing): 14–60. https://www.fine-americas.com/wp-content/uploads/2022/01/PGR_Guide_2022-2023.pdf. Retrieved 2022-04-06.
- ↑ Bai, Shuju; Chaney, William; Qi, Yadong (May 1, 2004). "Response of Cambial and Shoot Growth in Trees Treated with Paclobutrazol". Arboriculture & Urban Forestry 30 (3): 137–145. doi:10.48044/jauf.2004.017.
- ↑ Berova, Malgorzata; Zlatev, Zlatko (2000). "Physiological response and yield of paclobutrazol treated tomato plants (Lycopersicon esculentum Mill.)". Plant Growth Regulation 30 (2): 117. doi:10.1023/A:1006300326975.
- ↑ Pal, Sikander; Zhao, Jiangsan; Khan, Asif; Yadav, Narendra Singh; Batushansky, Albert; Barak, Simon; Rewald, Boris; Fait, Aaron et al. (December 22, 2016). "Paclobutrazol induces tolerance in tomato to deficit irrigation through diversified effects on plant morphology, physiology and metabolism". Scientific Reports 6 (1): 39321. doi:10.1038/srep39321. PMID 28004823. Bibcode: 2016NatSR...639321P.
- ↑ Grossi et al. (2005). "Effects of paclobutrazol on growth and fruiting characteristics of Pitanga ornamental pepper". Acta Horticulturae 683 (683): 333–336. doi:10.17660/ActaHortic.2005.683.41.
- ↑ McCullough, Patrick E.; Liu, Haibo; McCarty, Lambert B.; Whitwell, Ted (2005). "Physiological Response of TifEagle Bermudagrass to Paclobutrazol". HortScience 40: 224–226. doi:10.21273/HORTSCI.40.1.224.
- ↑ 20.0 20.1 Baris, Reuben D.; Cohen, Stuart Z.; Barnes, N. LaJan; Lam, Juleen; Ma, Qingli (March 28, 2010). "A quantitative analysis of over twenty years of golf course monitoring studies". Environmental Toxicology and Chemistry 29 (6): 1224–1236. doi:10.1002/etc.185. PMID 20821564.
- ↑ Kamran, Muhammad; Cui, Wenwen; Ahmad, Irshad; Meng, Xiangping; Zhang, Xudong; Su, Wennan; Chen, Junzhi; Ahmad, Shakeel et al. (2017-11-08). "Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize". Plant Growth Regulation (Springer) 84 (2): 317–332. doi:10.1007/s10725-017-0342-8. ISSN 0167-6903.
- ↑ Tekalign, T. (December 7, 2007). "Growth, Photosynthetic Efficiency, Rate of Transpiration, Lodging, and Grain Yield of Tef (Eragrostis Tef (Zucc.) Trotter) as Influenced by Stage and Rate of Paclobutrazol Application". East African Journal of Sciences 1 (1): 35–44. doi:10.4314/eajsci.v1i1.40339.
- ↑ "Stress Tolerant Rice". International Rice Research Institute. https://strasa.irri.org/.
- ↑ 24.0 24.1 24.2
- Liu, Qingquan; Luo, Le; Zheng, Luqing (2018-01-24). "Lignins: Biosynthesis and Biological Functions in Plants". International Journal of Molecular Sciences (MDPI) 19 (2): 335. doi:10.3390/ijms19020335. ISSN 1422-0067. PMID 29364145.
- Welker, Cassie; Balasubramanian, Vimal; Petti, Carloalberto; Rai, Krishan; DeBolt, Seth; Mendu, Venugopal (2015-07-27). "Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts". Energies (MDPI) 8 (8): 7654–7676. doi:10.3390/en8087654. ISSN 1996-1073.
- Shah, Adnan Noor; Tanveer, Mohsin; Rehman, Atique ur; Anjum, Shakeel Ahmad; Iqbal, Javaid; Ahmad, Riaz (2016-12-26). "Lodging stress in cereal—effects and management: an overview". Environmental Science and Pollution Research (Springer) 24 (6): 5222–5237. doi:10.1007/s11356-016-8237-1. ISSN 0944-1344. PMID 28025787.
- Shah, Liaqat; Yahya, Muhammad; Shah, Syed Mehar Ali; Nadeem, Muhammad; Ali, Ahmad; Ali, Asif; Wang, Jing; Riaz, Muhammad Waheed et al. (2019-08-28). "Improving Lodging Resistance: Using Wheat and Rice as Classical Examples". International Journal of Molecular Sciences (MDPI) 20 (17): 4211. doi:10.3390/ijms20174211. ISSN 1422-0067. PMID 31466256.
- Khobra, Rinki; Sareen, Sindhu; Meena, Braj Kishor; Kumar, Arvind; Tiwari, Vinod; Singh, G. P. (2019-01-01). "Exploring the traits for lodging tolerance in wheat genotypes: a review". Physiology and Molecular Biology of Plants (Springer) 25 (3): 589–600. doi:10.1007/s12298-018-0629-x. ISSN 0971-5894. PMID 31168225.
- Vavilala, Sirisha L.; Ghag, Siddhesh B.; D’Souza, Jacinta S. (2019). "Lignin: Understanding and Exploring Its Potential for Biofuel Production". Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts. Woodhead Publishing Series in Energy. Woodhead. pp. 165–186. doi:10.1016/b978-0-12-817941-3.00009-7. ISBN 9780128179413.
- ↑ 25.0 25.1 25.2 "Conclusion on the peer review of the pesticide risk assessment of the active substance paclobutrazol". EFSA Journal 8 (11): 1876. 2010. doi:10.2903/j.efsa.2010.1876.
- ↑
- Peng, Jinrong; Carol, Pierre; Richards, Donald E.; King, Kathryn E.; Cowling, Rachel J.; Murphy, George P.; Harberd, Nicholas P. (1997). "The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses". Genes & Development 11 (23): 3194–3205. doi:10.1101/gad.11.23.3194. PMID 9389651.
- Olszewski, Neil; Sun, Tai-ping; Gubler, Frank (2002). "Gibberellin Signaling". The Plant Cell (American Society of Plant Biologists (OUP)) 14 (suppl 1): S61–S80. doi:10.1105/tpc.010476. ISSN 1040-4651. PMID 12045270.
- ↑ Ueguchi-Tanaka, Miyako; Nakajima, Masatoshi; Motoyuki, Ashikari; Matsuoka, Makoto (2007-06-01). "Gibberellin Receptor and Its Role in Gibberellin Signaling in Plants". Annual Review of Plant Biology (Annual Reviews) 58 (1): 183–198. doi:10.1146/annurev.arplant.58.032806.103830. ISSN 1543-5008. PMID 17472566.
External links
- Paclobutrazol in the Pesticide Properties DataBase (PPDB)
 |