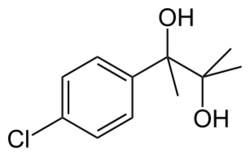
Chemistry:Phenaglycodol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C11H15ClO2 |
| Molar mass | 214.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Phenaglycodol (brand names Acalmid, Acalo, Alterton, Atadiol, Felixyn, Neotran, Pausital, Remin, Sedapsin, Sinforil, Stesil, Ultran)[1] is a drug described as a tranquilizer or sedative which has anxiolytic and anticonvulsant properties.[2][3] It is related pharmacologically to meprobamate, though it is not a carbamate.[4][5]
Synthesis

p-Chloroacetophenone and NaCN are reacted together to give the corresponding cyanohydrin (cf Strecker synthesis), CID:12439573. The cyano group is then hydrated in acid to the corresponding amide, p-chloroatrolactamide, CID:15255544 (4). The amide group is then further hydrolyzed with a 2nd equivalent of water in concentrated lye to p-chloroatrolactic acid, [4445-13-0] (5). Esterification to Ethyl p-chloroatrolactate [100126-96-3](6). Finally, nucleophilic addition a couple of equivalents of MeMgI are added to the ester give Phenaglycodol (7) crystals.

A mixed Pinacol coupling rxn between 4-chloroacetophenone [99-91-2] and acetone with magnesium activated with a small amount of trimethylsilyl chloride gave a 40% yield of phenglycodol.
Notes
- See "Novel trifluoromethyl derivatives of substituted diols" U.S. Patent 3,134,819 also.
- A Pinacol rearrangement occurs in acidic water:[9]
See also
References
- ↑ Psychotropic drugs and related compounds. National Institute of Mental Health; [for sale by the Supt. of Docs., U.S. Govt. Print. Off., Washington. 1972. ISBN 9780080255101. https://books.google.com/books?id=t-dsAAAAMAAJ.
- ↑ Anticonvulsants. Elsevier. 19 July 2013. pp. 578–. ISBN 978-0-323-14395-0. https://books.google.com/books?id=u-qOecoMmqMC&pg=PA578.
- ↑ Clinical Management of Poisoning and Drug Overdose. Saunders. 1983. ISBN 978-0-7216-4447-9. https://archive.org/details/clinicalmanageme00hadd.
- ↑ Pharmacology in Medicine: A Collaborative Textbook. McGraw-Hill. 1958. https://books.google.com/books?id=FfdsAAAAMAAJ.
- ↑ Pharmacology; the nature, action and use of drugs. Saunders. 1961. https://books.google.com/books?id=i_5sAAAAMAAJ.
- ↑ Jack Mills "2-chlorophenyl-3-methyl-2, 3-butanediols" U.S. Patent 2,812,363 (1957 to Eli Lilly Co.)
- ↑ Mills, Jack, "Improvements in or relating to novel substituted butanediol", GB patent 788896, published 1958-01-08, assigned to Eli Lilly & Co.
- ↑ Maekawa, Hirofumi; Yamamoto, Yoshimasa; Shimada, Hisashi; Yonemura, Kazuaki; Nishiguchi, Ikuzo (2004). "Mg-promoted mixed pinacol coupling". Tetrahedron Letters. 45 (20): 3869–3872. doi:10.1016/j.tetlet.2004.03.109.
- ↑ Murphy, Hubert W. (1964). "Pinacol Rearrangement of Phenaglycodol I". Journal of Pharmaceutical Sciences. 53 (3): 298–301. doi:10.1002/jps.2600530311.
 |
