Chemistry:Titanium tetrachloride

| |

| |
| Names | |
|---|---|
| IUPAC name
Titanium(IV) chloride
| |
| Other names
Titanium tetrachloride
Tetrachlorotitanium | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | Titanium+tetrachloride |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1838 |
| |
| |
| Properties | |
| TiCl 4 | |
| Molar mass | 189.679 g/mol |
| Appearance | Colourless liquid |
| Odor | penetrating acid odor |
| Density | 1.726 g/cm3 |
| Melting point | −24.1 °C (−11.4 °F; 249.1 K) |
| Boiling point | 136.4 °C (277.5 °F; 409.5 K) |
| reacts (exothermic hydrolysis)[1] | |
| Solubility | soluble in dichloromethane,[2] toluene,[3] pentane[4] |
| Vapor pressure | 1.3 kPa (20 °C) |
| −54.0·10−6 cm3/mol | |
Refractive index (nD)
|
1.61 (10.5 °C) |
| Viscosity | 827 μPa s |
| Structure | |
| Tetragonal | |
| Tetrahedral | |
| 0 D | |
| Thermochemistry | |
Std molar
entropy (S |
355 J·mol−1·K−1[5] |
Std enthalpy of
formation (ΔfH⦵298) |
−763 kJ·mol−1[5] |
| Hazards[6] | |
| Main hazards | Toxic, corrosive, reacts with water to release HCl |
| Safety data sheet | MSDS |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H314, H317, H330, H335, H370, H372 | |
| P280, P301+330+331, P304+340, P305+351+338, P308+310Script error: No such module "Preview warning".Category:GHS errors | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Titanium(IV) bromide Titanium(IV) fluoride Titanium(IV) iodide |
Other cations
|
Hafnium(IV) chloride Zirconium(IV) chloride |
Related compounds
|
Titanium(II) chloride Titanium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Titanium tetrachloride is the inorganic compound with the formula TiCl
4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl
4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide (TiO
2) and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as “tickle” or “tickle 4”, as a phonetic representation of the symbols of its molecular formula (TiCl
4).[7][8]
Properties and structure
TiCl
4 is a dense, colourless liquid, although crude samples may be yellow or even red-brown. It is one of the rare transition metal halides that is a liquid at room temperature, VCl
4 being another example. This property reflects the fact that molecules of TiCl
4 weakly self-associate. Most metal chlorides are polymers, wherein the chloride atoms bridge between the metals. Its melting point is similar to that of CCl
4.[9][10]
Ti4+ has a "closed" electronic shell, with the same number of electrons as the noble gas argon. The tetrahedral structure for TiCl
4 is consistent with its description as a d0 metal center (Ti4+) surrounded by four identical ligands. This configuration leads to highly symmetrical structures, hence the tetrahedral shape of the molecule. TiCl
4 adopts similar structures to TiBr
4 and TiI
4; the three compounds share many similarities. TiCl
4 and TiBr
4 react to give mixed halides TiCl
4-xBr
x, where x = 0, 1, 2, 3, 4. Magnetic resonance measurements also indicate that halide exchange is also rapid between TiCl
4 and VCl
4.[11]
TiCl
4 is soluble in toluene and chlorocarbons. Certain arenes form complexes of the type [(C
6R
6)TiCl
3]+
.[12] TiCl
4 reacts exothermically with donor solvents such as THF to give hexacoordinated adducts.[13] Bulkier ligands (L) give pentacoordinated adducts TiCl
4L.
Production
TiCl
4 is produced by the chloride process, which involves the reduction of titanium oxide ores, typically ilmenite (FeTiO
3), with carbon under flowing chlorine at 900 °C. Impurities are removed by distillation.[10]
- 2 FeTiO
3 + 7 Cl
2 + 6 C → 2 TiCl
4 + 2 FeCl
3 + 6 CO
The coproduction of FeCl
3 is undesirable, which has motivated the development of alternative technologies. Instead of directly using ilmenite, "rutile slag" is used. This material, an impure form of TiO
2, is derived from ilmenite by removal of iron, either using carbon reduction or extraction with sulfuric acid. Crude TiCl
4 contains a variety of other volatile halides, including vanadyl chloride (VOCl
3), silicon tetrachloride (SiCl
4), and tin tetrachloride (SnCl
4), which must be separated.[10]
Applications
Production of titanium metal
The world's supply of titanium metal, about 250,000 tons per year, is made from TiCl
4. The conversion involves the reduction of the tetrachloride with magnesium metal. This procedure is known as the Kroll process:[14]
- 2 Mg + TiCl
4 → 2 MgCl
2 + Ti
In the Hunter process, liquid sodium is the reducing agent instead of magnesium.[15]
Production of titanium dioxide
Around 90% of the TiCl
4 production is used to make the pigment titanium dioxide (TiO
2). The conversion involves hydrolysis of TiCl
4, a process that forms hydrogen chloride:[14]
- TiCl
4 + 2 H
2O → TiO
2 + 4 HCl
In some cases, TiCl
4 is oxidised directly with oxygen:
- TiCl
4 + O
2 → TiO
2 + 2 Cl
2
Smoke screens
It has been used to produce smoke screens since it produces a heavy, white smoke that has little tendency to rise. "Tickle" was the standard means of producing on-set smoke effects for motion pictures, before being phased out in the 1980s due to concerns about hydrated HCl's effects on the respiratory system.[16]
Chemical reactions
Titanium tetrachloride is a versatile reagent that forms diverse derivatives including those illustrated below.[17]
A characteristic reaction of TiCl
4 is its easy hydrolysis, signaled by the release of HCl vapors and titanium oxides and oxychlorides. Titanium tetrachloride has been used to create naval smokescreens, as the hydrochloric acid aerosol and titanium dioxide that is formed scatter light very efficiently. This smoke is corrosive, however.[10]
Alcohols react with TiCl
4 to give alkoxides with the formula [Ti(OR)
4]
n (R = alkyl, n = 1, 2, 4). As indicated by their formula, these alkoxides can adopt complex structures ranging from monomers to tetramers. Such compounds are useful in materials science as well as organic synthesis. A well known derivative is titanium isopropoxide, which is a monomer. Titanium bis(acetylacetonate)dichloride results from treatment of titanium tetrachloride with excess acetylacetone:[18]
Organic amines react with TiCl
4 to give complexes containing amido (R
2N−
-containing) and imido (RN2−-containing) complexes. With ammonia, titanium nitride is formed. An illustrative reaction is the synthesis of tetrakis(dimethylamido)titanium Ti(N(CH
3)
2)
4, a yellow, benzene-soluble liquid:[19] This molecule is tetrahedral, with planar nitrogen centers.[20]
- 4 LiN(CH
3)
2 + TiCl
4 → 4 LiCl + Ti(N(CH
3)
2)
4
Complexes with simple ligands
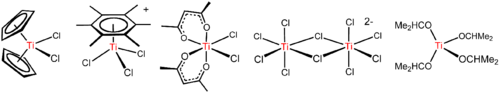
TiCl
4 is a Lewis acid as implicated by its tendency to hydrolyze. With the ether THF, TiCl
4 reacts to give yellow crystals of TiCl
4(THF)
2. With chloride salts, TiCl
4 reacts to form sequentially [Ti
2Cl
9]−
, [Ti
2Cl
10]2− (see figure above), and [TiCl
6]2−.[21] The reaction of chloride ions with TiCl
4 depends on the counterion. [N(CH
2CH
2CH
2CH
3)
4]Cl and TiCl
4 gives the pentacoordinate complex [N(CH
2CH
2CH
2CH
3)
4][TiCl
5], whereas smaller [N(CH
2CH
3)
4]+
gives [N(CH
2CH
3)
4]
2[Ti
2Cl
10]. These reactions highlight the influence of electrostatics on the structures of compounds with highly ionic bonding.
Redox
Reduction of TiCl
4 with aluminium results in one-electron reduction. The trichloride (TiCl
3) and tetrachloride have contrasting properties: the trichloride is a colored solid, being a coordination polymer, and is paramagnetic. When the reduction is conducted in THF solution, the Ti(III) product converts to the light-blue adduct TiCl
3(THF)
3.
Organometallic chemistry
The organometallic chemistry of titanium typically starts from TiCl
4. An important reaction involves sodium cyclopentadienyl to give titanocene dichloride, TiCl
2(C
5H
5)
2. This compound and many of its derivatives are precursors to Ziegler–Natta catalysts. Tebbe's reagent, useful in organic chemistry, is an aluminium-containing derivative of titanocene that arises from the reaction of titanocene dichloride with trimethylaluminium. It is used for the "olefination" reactions.[17]
Arenes, such as C
6(CH
3)
6 react to give the piano-stool complexes [Ti(C
6R
6)Cl
3]+
(R = H, CH
3; see figure above). This reaction illustrates the high Lewis acidity of the TiCl+
3 entity, which is generated by abstraction of chloride from TiCl
4 by AlCl
3.[12]
Reagent in organic synthesis
TiCl
4 finds occasional use in organic synthesis, capitalizing on its Lewis acidity, its oxophilicity, and the electron-transfer properties of its reduced titanium halides. It is used in the Lewis acid catalysed aldol addition[22] Key to this application is the tendency of TiCl
4 to activate aldehydes (RCHO) by formation of adducts such as (RCHO)TiCl
4OC(H)R.[23]
Toxicity and safety considerations
Hazards posed by titanium tetrachloride generally arise from its reaction with water that releases hydrochloric acid, which is severely corrosive itself and whose vapors are also extremely irritating. TiCl
4 is a strong Lewis acid, which exothermically forms adducts with even weak bases such as THF and water.
References
- ↑ Eremenko, B. V.; Bezuglaya, T. N.; Savitskaya, A. N.; Malysheva, M. L.; Kozlov, I. S.; Bogodist, L. G. (2001). "Stability of Aqueous Dispersions of the Hydrated Titanium Dioxide Prepared by Titanium Tetrachloride Hydrolysis". Colloid Journal 63 (2): 173–178. doi:10.1023/A:1016673605744.
- ↑ "titanium(IV) chloride, 1M soln. in dichloromethane". https://www.alfa.com/en/catalog/H31830/.
- ↑ "Titanium(IV) chloride solution 1.0 M in toluene". https://www.sigmaaldrich.com/catalog/product/sigald/345695.
- ↑ Butts, Edward H De. "patent US3021349A". https://patents.google.com/patent/US3021349.
- ↑ 5.0 5.1 Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton-Mifflin. p. A23. ISBN 978-0-618-94690-7.
- ↑ "Classifications - CL Inventory". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/3837.
- ↑ [1] American Chemistry Council – "Titanium Tetrachloride: Stepping Stone to Amazing Technology"
- ↑ "Archived copy". http://avogadro.chem.iastate.edu/MSDS/TiCl4.htm. Iowa State University – "Chemistry Material Safety Data Sheets"
- ↑ Earnshaw, A.; Greenwood, N. (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann.
- ↑ 10.0 10.1 10.2 10.3 Heinz Sibum; Volker Güther; Oskar Roidl; Fathi Habashi; Hans Uwe Wolf; Carsten Siemers (2017). "Titanium, Titanium Alloys, and Titanium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_095.pub2. ISBN 978-3527306732.
- ↑ Webb, S. P.; Gordon, M. S. (1999). "Intermolecular Self-Interactions of the Titanium Tetrahalides TiX4 (X = F, Cl, Br)". J. Am. Chem. Soc. 121 (11): 2552–2560. doi:10.1021/ja983339i. https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=1355&context=chem_pubs.
- ↑ 12.0 12.1 Calderazzo, F.; Ferri, I.; Pampaloni, G.; Troyanov, S. (1996). "η6-Arene Derivatives of Titanium(IV), Zirconium(IV) and Hafnium(IV)". J. Organomet. Chem. 518 (1–2): 189–196. doi:10.1016/0022-328X(96)06194-3.
- ↑ Manzer, L. E. (1982). Tetrahydrofuran Complexes of Selected Early Transition Metals. Inorganic Syntheses. 21. pp. 135–40. doi:10.1002/9780470132524.ch31. ISBN 978-0-470-13252-4.
- ↑ 14.0 14.1 Völz, Hans G. (2006). "Inorganic Pigments". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.n20_n04. ISBN 978-3527306732.
- ↑ Schaschke, Carl (2014) (in en). Oxford University Press. doi:10.1093/acref/9780199651450.001.0001. ISBN 978-0-19-965145-0. http://www.oxfordreference.com/view/10.1093/acref/9780199651450.001.0001/acref-9780199651450-e-1447.
- ↑ The Royal Navy at War (DVD). London: Imperial War Museum. 2005.
- ↑ 17.0 17.1 "Organotitanium Reagents in Organic Synthesis (Reactivity and Structure Concepts in Organic Chemistry, Vol 24)" Manfred T. Reetz 1986 ISBN:0-387-15784-0
- ↑ Wilkie, C. A.; Lin, G.; Haworth, D. T. (1979). "Cis ‐[Dihalobis(2,4‐Pentaedionato)Titanium(IV)] Complexes". Inorganic Syntheses. 19. 145–148. doi:10.1002/9780470132500.ch33. ISBN 9780470132500.
- ↑ Bradey, D. C.; Thomas, M. (1960). "Some Dialkylamino-derivatives of Titanium and Zirconium". J. Chem. Soc.: 3857–3861. doi:10.1039/JR9600003857.
- ↑ M. E. Davie; T. Foerster; S. Parsons; C. Pulham; D. W. H. Rankin; B. A. Smart (2006). "The Crystal Structure of Tetrakis(dimethylamino)titanium(IV)". Polyhedron 25 (4): 923–929. doi:10.1016/j.poly.2005.10.019.
- ↑ Creaser, C. S.; Creighton, J. A. (1975). "Pentachloro- and Pentabromotitanate(IV) ions". Dalton Trans. (14): 1402–1405. doi:10.1039/DT9750001402.
- ↑ Mariappan Periasamy (2002): "New synthetic methods using the TiCl4-NR3 reagent system", Arkivoc, p. 151-166.
- ↑ Gundersen, L.-L.; Rise, F.; Undheim, K. (2004). "Titanium(IV) chloride". in Paquette, L.. Encyclopedia of Reagents for Organic Synthesis. New York, NY: J. Wiley & Sons.
General reading
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego, CA: Academic Press. ISBN 978-0-12-352651-9.
External links
- Titanium tetrachloride: Health Hazard Information
- NIST Standard Reference Database
- ChemSub Online: Titanium tetrachloride
 |