Chemistry:Praseodymium(III) carbonate
| |
| Names | |
|---|---|
| IUPAC name
Praseodymium(III) carbonate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
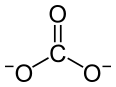
| Pr2(CO3)3 | |
| Molar mass | 461.849 (anhydrous) 605.977 (octahydrate) |
| Appearance | green crystals (octahydrate) [1] |
| insoluble (1.99×10−6mol/L) [2] | |
| Related compounds | |
Other anions
|
Praseodymium(III) chloroacetate Praseodymium(III) sulfate Praseodymium(III) nitrate |
Other cations
|
cerium carbonate neodymium carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Praseodymium(III) carbonate is an inorganic compound, with a chemical formula of Pr2(CO3)3. The anhydrous form is olive green, and many of its hydrates such as heptahydrate and octahydrate are known.[3] They are all insoluble in water.[2]
Preparation
Praseodymium(III) carbonate can be obtained by the hydrolysis of praseodymium(III) chloroacetate:[2][4]
- 2 Pr(C2Cl3O2)3 + 3 H2O → Pr2(CO3)3 + 6 CHCl3 + 3 CO2
It can also be obtained by reacting sodium bicarbonate saturated with carbon dioxide with a praseodymium chloride solution.[4]
Chemical properties
Praseodymium(III) carbonate is soluble in acids, and emits carbon dioxide:[5]
- Pr2(CO3)3 + 6 H+ → 2 Pr3+ + 3 H2O + 3 CO2↑
However, it is insoluble in water.[2]
Other compounds
Praseodymium(III) carbonate forms compounds with N2H4, such as Pr2(CO3)3•12N2H4•5H2O which is a pale green crystal that is slightly soluble in water but insoluble in benzene, with d20°C = 1.873 g/cm3.[6]
References
- ↑ 《化学化工物性数据手册》 (无机卷) . 化学工业出版社. P320. 7.2 碳酸盐. ISBN:7-5025-3591-8
- ↑ 2.0 2.1 2.2 2.3 《无机化学丛书》. 第七卷 钪 稀土元素. 易宪武 黄春晖 等编.科学出版社. P174. 碳酸盐. ISBN:978-7-03-030574-9
- ↑ Rare earth elements: Main volume, Phần 3 (Leopold Gmelin; Verlag Chemie, 1994), page 22; 68. Retrieved 4 Feb 2021.
- ↑ 4.0 4.1 冯天泽. 稀土碳酸盐的制法、性质和组成. 《稀土》. 1989年第3期. pp.45~49
- ↑ PubChem. "Praseodymium Carbonate Octahydrate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/25022096.
- ↑ Uchenye zapiski: Serii︠a︡ khimicheskikh nauk (S.M. Kirov adyna Azărbai̐jan Dȯvlăt Universiteti; 1977), page 37. Retrieved 7 Feb 2021.
| H2CO3 | He | ||||||||||||||||
| Li2CO3, LiHCO3 |
BeCO3 | B | C | (NH4)2CO3, NH4HCO3 |
O | F | Ne | ||||||||||
| Na2CO3, NaHCO3, Na3H(CO3)2 |
MgCO3, Mg(HCO3)2 |
Al2(CO3)3 | Si | P | S | Cl | Ar | ||||||||||
| K2CO3, KHCO3 |
CaCO3, Ca(HCO3)2 |
Sc | Ti | V | Cr | MnCO3 | FeCO3 | CoCO3 | NiCO3 | CuCO3 | ZnCO3 | Ga | Ge | As | Se | Br | Kr |
| Rb2CO3 | SrCO3 | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2CO3 | CdCO3 | In | Sn | Sb | Te | I | Xe |
| Cs2CO3, CsHCO3 |
BaCO3 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl2CO3 | PbCO3 | (BiO)2CO3 | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La2(CO3)3 | Ce2(CO3)3 | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UO2CO3 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |

