Chemistry:Uranyl carbonate
| |
 Uranyl carbonate
| |
| Names | |
|---|---|
| IUPAC name
Uranium carbonate
| |
| Other names
Uranium Carbonate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| UO2(CO3) | |
| Molar mass | 330 g/mol |
| Density | 5.7 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
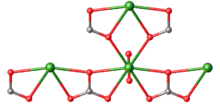
Uranyl carbonate refers to the inorganic compound with the formula UO2CO3. Also known by its mineral name rutherfordine, this material consists of uranyl (UO2+
2) and carbonate (CO2−
3). Like most uranyl salts, the compound is a polymeric, each uranium(VI) center being bonded to eight oxygen atoms.[1] Hydrolysis products of rutherfordine are also found in both the mineral and organic fractions of coal and its fly ash and is the main component of uranium in mine tailing seepage water.[2]
Uranyl carbonates as a class of materials
Many uranyl carbonates exist, rutherfordine being the simplest stoichiometry. Most uranyl carbonates contain additional components including water and diverse anions and cations.[3]
A common method for concentrating uranium from a solution uses solutions of uranyl carbonates, which are passed through a resin bed where the complex ions are transferred to the resin by ion exchange with a negative ion like chloride. After build-up of the uranium complex on the resin, the uranium is eluted with a salt solution and the uranium is precipitated in another process.[citation needed]
Uranyl carbonate minerals
Uranyl carbonates include:[citation needed]
- Andersonite (hydrated sodium calcium uranyl carbonate)
- Astrocyanite-(Ce) (hydrated copper cerium neodymium lanthanum praseodymium samarium calcium yttrium uranyl carbonate hydroxide)
- Bayleyite (hydrated magnesium uranyl carbonate)
- Bijvoetite-(Y) (hydrated yttrium dysprosium uranyl carbonate hydroxide)
- Fontanite (hydrated calcium uranyl carbonate)
- Grimselite (hydrated potassium sodium uranyl carbonate)
- Joliotite (hydrated uranyl carbonate)
- Liebigite (hydrated calcium uranyl carbonate)
- Mckelveyite-(Y) (hydrated barium sodium calcium uranium yttrium carbonate)
- Metazellerite (hydrated calcium uranyl carbonate)
- Rabbittite (hydrated calcium magnesium uranyl carbonate hydroxide)
- Roubaultite (copper uranyl carbonate oxide hydroxide)
- Rutherfordine (uranyl carbonate)
- Schröckingerite (hydrated sodium calcium uranyl sulfate carbonate fluoride)
- Shabaite (hydrated copper cerium neodymium lanthanum praseodymium samarium calcium yttrium uranyl carbonate hydroxide)
- Sharpite (hydrated calcium uranyl carbonate hydroxide)
- Swartzite (hydrated calcium magnesium uranyl carbonate)
- Voglite (hydrated calcium copper uranyl carbonate)
- Wyartite (hydrated calcium uranyl carbonate hydroxide)
- Widenmannite (lead uranyl carbonate)
- Zellerite (hydrated calcium uranyl carbonate)
- Znucalite (hydrated calcium zinc uranyl carbonate hydroxide)
References
- ↑ Finch, R. J.; Cooper, M. A.; Hawthorne, F. C.; Ewing, R. C. (1999). "Refinement of the Crystal Structure of Rutherfordine". The Canadian Mineralogist 37 (4): 929–938.
- ↑ Ivanovich, M.; Fröhlich, K.; Hendry, M.J. (1991). "Uranium-series radionuclides in fluids and solids, Milk River aquifer, Alberta, Canada". Applied Geochemistry 6 (4): 405–418. doi:10.1016/0883-2927(91)90040-V. Bibcode: 1991ApGC....6..405I.
- ↑ Amayri, Samer; Reich, Tobias; Arnold, Thuro; Geipel, Gerhard; Bernhard, Gert (2005). "Spectroscopic Characterization of Alkaline Earth Uranyl Carbonates". Journal of Solid State Chemistry 178 (2): 567–577. doi:10.1016/j.jssc.2004.07.050. Bibcode: 2005JSSCh.178..567A.
- "Radioactive Elements in Coal and Fly Ash: Abundance, Forms, and Environmental Significance". Fact Sheet FS-163-97. U.S. Geological Survey. October 1997. http://pubs.usgs.gov/fs/1997/fs163-97/FS-163-97.pdf.
- "Ion-exchange". U.S. Nuclear Regulatory Commission. 6 October 2011. https://www.nrc.gov/reading-rm/basic-ref/glossary/ion-exchange.html.
- Flury, Markus; Harsh, James B. (2000). "Remediation of Uranium Contaminated Mine Waste". State of Washington Water Research Center Report WRR-04. State of Washington Water Research Center. http://www.swwrc.wsu.edu/reports/WRR-04.pdf.
- "The Uranyl Carbonates". Mineral Gallery. Amethyst Galleries. http://www.galleries.com/Carbonates#uranyl.
Carbonates H2CO3 He Li2CO3,
LiHCO3BeCO3 B C (NH4)2CO3,
NH4HCO3O F Ne Na2CO3,
NaHCO3,
Na3H(CO3)2MgCO3,
Mg(HCO3)2Al2(CO3)3 Si P S Cl Ar K2CO3,
KHCO3CaCO3,
Ca(HCO3)2Sc Ti V Cr MnCO3 FeCO3 CoCO3 NiCO3 CuCO3 ZnCO3 Ga Ge As Se Br Kr Rb2CO3 SrCO3 Y Zr Nb Mo Tc Ru Rh Pd Ag2CO3 CdCO3 In Sn Sb Te I Xe Cs2CO3,
CsHCO3BaCO3 Hf Ta W Re Os Ir Pt Au Hg Tl2CO3 PbCO3 (BiO)2CO3 Po At Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og ↓ La2(CO3)3 Ce2(CO3)3 Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Ac Th Pa UO2CO3 Np Pu Am Cm Bk Cf Es Fm Md No Lr
 Original source: https://en.wikipedia.org/wiki/Uranyl carbonate. Read more
Original source: https://en.wikipedia.org/wiki/Uranyl carbonate. Read more
