Chemistry:2-Methyl-2-pentanol
From HandWiki
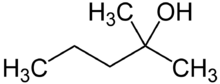
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpentan-2-ol | |
| Other names
2-Methyl-2-pentanol
Dimethyl propyl carbinol Dimethylbutanol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2560 |
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.177 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8350 g/cm3 at 20 °C |
| Melting point | −103 °C (−153 °F; 170 K) |
| Boiling point | 121.1 °C (250.0 °F; 394.2 K) |
| 33 g/L | |
| Solubility | soluble[vague] in ethanol, diethyl ether |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
| Related compounds | |
Related compounds
|
Hexanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Methyl-2-pentanol (IUPAC name: 2-methylpentan-2-ol) is an organic chemical compound. It can be added to a gas chromatograph to help distinguish between branched compounds, especially alcohols.[2] Its presence in urine can be used to test for exposure to 2-methylpentane.[3] As with many other short-chain alcohols, 2-methyl-2-pentanol can produce intoxication and sedative effects similar to those of ethanol, though it is more irritating to mucous membranes and generally more toxic to the body.[4]
See also
- 2-Methyl-2-butanol
- 3-Methyl-3-pentanol
References
- ↑ Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. 1998. pp. 3–398, 8-106. ISBN 0-8493-0594-2.
- ↑ Guiochon G, Guillemin CL (1988). Quantitative gas chromatography: for laboratory analyses and on-line process control. Elsevier. pp. 518. ISBN 978-0-444-42857-8. https://books.google.com/books?id=TZcWf2LD2_MC&pg=PA518. Retrieved 2010-01-22.
- ↑ Industrial chemical exposure: guidelines for biological monitoring. CRC Press. 2001. pp. 190. ISBN 978-1-56670-545-5. https://books.google.com/books?id=8pViePEG27sC&pg=PA190. Retrieved 2010-01-22.
- ↑ "Ethanol and the GABA A receptor-gated chloride ion channel.". Neuropharmacology of Ethanol. Boston, MA.: Birkhäuser. 1991. pp. 49–76. doi:10.1007/978-1-4757-1305-3_3. ISBN 978-1-4757-1307-7.
 |

