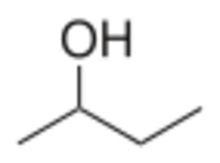
Chemistry:2-Butanol
| |
| Names | |
|---|---|
| Preferred IUPAC name
Butan-2-ol[2] | |
| Other names | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 773649 1718764 (R) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 1686 396584 (R) | |
| MeSH | 2-butanol |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 1120 |
| |
| |
| Properties | |
| C4H10O | |
| Molar mass | 74.123 g·mol−1 |
| Density | 0.808 g cm−3 |
| Melting point | −115 °C; −175 °F; 158 K |
| Boiling point | 98 to 100 °C; 208 to 212 °F; 371 to 373 K |
| 290 g/L[3] | |
| log P | 0.683 |
| Vapor pressure | 1.67 kPa (at 20 °C) |
| Acidity (pKa) | 17.6 [4] |
| −5.7683×10−5 cm3 mol−1 | |
Refractive index (nD)
|
1.3978 (at 20 °C) |
| Thermochemistry | |
Heat capacity (C)
|
197.1 J K−1 mol−1 |
Std molar
entropy (S |
213.1 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−343.3 to −342.1 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−2.6611 to −2.6601 MJ mol−1 |
| Hazards | |
| Safety data sheet | inchem.org |
| GHS pictograms |  
|
| GHS Signal word | WARNING |
| H226, H319, H335, H336 | |
| P261, P305+351+338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 22 to 27 °C (72 to 81 °F; 295 to 300 K) |
| 405 °C (761 °F; 678 K) | |
| Explosive limits | 1.7–9.8% |
| Lethal dose or concentration (LD, LC): | |
LCLo (lowest published)
|
16,000 ppm (rat, 4 hr) 10,670 ppm (mouse, 3.75 hr) 16,000 ppm (mouse, 2.67 hr)[5] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 150 ppm (450 mg/m3)[5] |
REL (Recommended)
|
TWA 100 ppm (305 mg/m3) ST 150 ppm (455 mg/m3)[5] |
IDLH (Immediate danger)
|
2000 ppm[5] |
| Related compounds | |
Related butanols
|
n-Butanol Isobutanol tert-Butanol |
Related compounds
|
Butanone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
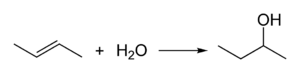
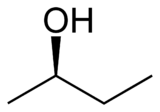
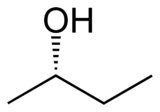
Butan-2-ol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. Its structural isomers are 1-butanol, isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-butan-2-ol and (S)-(+)-butan-2-ol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.
This secondary alcohol is a flammable, colorless liquid that is soluble in three parts water and completely miscible with organic solvents. It is produced on a large scale, primarily as a precursor to the industrial solvent methyl ethyl ketone.
 |
|
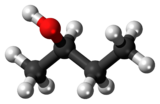 |
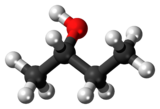
|
| (R)-(−)-2-butanol | (S)-(+)-2-butanol |
Manufacture and applications
Butan-2-ol is manufactured industrially by the hydration of 1-butene or 2-butene:
Sulfuric acid is used as a catalyst for this conversion.[6]
In the laboratory it can be prepared via Grignard reaction by reacting ethylmagnesium bromide with acetaldehyde in dried diethyl ether or tetrahydrofuran.
Although some butan-2-ol is used as a solvent, it is mainly converted to butanone (methyl ethyl ketone, MEK), an important industrial solvent and found in many domestic cleaning agents and paint removers. Though most paint removers have ceased using MEK in their products due to health concerns and new laws. Volatile esters of butan-2-ol have pleasant aromas and are used in small amounts as perfumes or in artificial flavors.[6]
Solubility
The listed solubility of butan-2-ol is often incorrect,[3] including some of the most well-known references such as the Merck Index, the CRC Handbook of Chemistry and Physics, and Lange's Handbook of Chemistry. Even the International Programme on Chemical Safety lists the wrong solubility. This widespread error originated because of Beilstein's Handbuch der Organischen Chemie (Handbook of Organic Chemistry). This work cites a false solubility of 12.5 g/100 g water. Many other sources used this solubility, which has snowballed into a widespread error in the industrial world. The correct data (35.0 g/100 g at 20 °C, 29 g/100 g at 25 °C, and 22 g/100 g at 30 °C) were first published in 1886 by Alexejew and then similar data was reported by other scientists including Dolgolenko and Dryer in 1907 and 1913, respectively.[3]
Precautions
Like other butanols, butan-2-ol has low acute toxicity. The -1">50 is 4400 mg/kg (rat, oral).[6]
Several explosions have been reported[7][8][9] during the conventional distillation of 2-butanol, apparently due to the buildup of peroxides with the boiling point higher than that of pure alcohol (and therefore concentrating in the still pot during distillation). As alcohols, unlike ethers, are not widely known to be capable of forming peroxide impurities, the danger is likely to be overlooked. 2-Butanol is in Class B Peroxide Forming Chemicals[10]
References
- ↑ "Alcohols Rule C-201.1". Nomenclature of Organic Chemistry (The IUPAC 'Blue Book'), Sections A, B, C, D, E, F, and H. Oxford: Pergamon Press. 1979. "Designations such as isopropanol, sec-butanol, and tert-butanol are incorrect because there are no hydrocarbons isopropane, sec-butane, and tert-butane to which the suffix "-ol" can be added; such names should be abandoned. Isopropyl alcohol, sec-butyl alcohol, and tert-butyl alcohol are, however, permissible (see Rule C-201.3) because the radicals isopropyl, sec-butyl, and tert-butyl do exist"
- ↑ "2-butanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6568.
- ↑ 3.0 3.1 3.2 Alger, Donald B. (November 1991). "The water solubility of butan-2-ol: A widespread error". Journal of Chemical Education 68 (11): 939. doi:10.1021/ed068p939.1. Bibcode: 1991JChEd..68..939A.
- ↑ Serjeant, E.P., Dempsey B.; Ionisation Constants of Organic Acids in Aqueous Solution. International Union of Pure and Applied Chemistry (IUPAC). IUPAC Chemical Data Series No. 23, 1979. New York, New York: Pergamon Press, Inc., p. 989
- ↑ 5.0 5.1 5.2 5.3 NIOSH Pocket Guide to Chemical Hazards. "#0077". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0077.html.
- ↑ 6.0 6.1 6.2 Hahn, Heinz-Dieter; Dämbkes, Georg; Rupprich, Norbert (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH..
- ↑ Doyle, R. R. (1986). "2-Butanol safety warning". Journal of Chemical Education 63 (2): 186. doi:10.1021/ed063p186.2. Bibcode: 1986JChEd..63..186D.
- ↑ Peterson, Donald (11 May 1981). "Letters: Explosion of 2-butanol". Chemical & Engineering News 59 (19): 3. doi:10.1021/cen-v059n019.p002.
- ↑ Watkins, Kenneth W. (May 1984). "Demonstration hazard". Journal of Chemical Education 61 (5): 476. doi:10.1021/ed061p476.3. Bibcode: 1984JChEd..61..476W.
- ↑ "Classification List of Peroxide Forming Chemicals". https://ehs.ucsc.edu/lab-safety-manual/specialty-chemicals/peroxide-formers-list.html.
External links
- International Chemical Safety Card 0112
- NIOSH Pocket Guide to Chemical Hazards. "#0077". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0077.html.
- IPCS Environmental Health Criteria 65: Butanols: four isomers
- IPCS Health and Safety Guide 4: 2-Butanol
 |