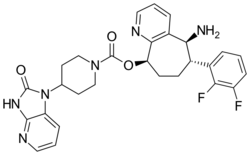
Chemistry:Rimegepant
 | |
| Clinical data | |
|---|---|
| Trade names | Nurtec ODT, Vydura |
| Other names | BHV-3000, BMS-927711 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620031 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Calcitonin gene-related peptide receptor antagonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C28H28F2N6O3 |
| Molar mass | 534.568 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rimegepant, sold under the brand name Nurtec ODT among others, is a medication used for the acute treatment of migraine with or without aura in adults and the prophylactic/ preventive treatment of episodic migraine in adults.[3][5] It is taken by mouth to dissolve on or under the tongue.[3] It works by blocking CGRP receptors.[6]
In the United States, rimegepant was approved for treating acute migraine in February 2020,[7] and its approval was extended to preventing episodic migraine in June 2021.[3] It is produced and marketed by Pfizer.[8] In March 2021, rimegepant was approved for medical use in the United Arab Emirates and in Israel.[9][10][11]
Medical uses
Rimegepant is indicated for the treatment of acute migraine with or without aura in adults and for the preventative treatment of episodic migraine in adults.[3][5][4]
Mechanism of action
Rimegepant is a small molecule calcitonin gene-related peptide (CGRP) receptor antagonist.[6]
History
Rimegepant was developed by Biohaven Pharmaceuticals, which markets the drug in the United States after receiving FDA approval in February 2020.[7][12] Approval was based on evidence from one clinical trial of 1,351 subjects with migraine headaches.[5]
The favorable results of rimegepant's first clinical trials were followed by ongoing studies with other gepants. In this way, the second generation of gepants emerged, and it includes rimegepant (BHV-3000, BMS-927711), ubrogepant (MK-1602), and atogepant (AGN-241689, MK-8031). These drugs revealed a low number of side effects, with excellent bioavailability due to molecular modifications.[13]
Society and culture
Legal status
On 24 February 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Vydura, intended for the prophylaxis and acute treatment of migraine.[14] The applicant for this medicinal product is Biohaven Pharmaceutical Ireland DAC.[14] Rimegepant was approved for medical use in the European Union in April 2022.[4][15]
References
- ↑ 1.0 1.1 "Nurtec ODT". 8 August 2023. https://www.tga.gov.au/resources/auspmd/nurtec-odt.
- ↑ "Nurtec ODT (Pfizer Australia Pty Ltd)". 22 August 2023. https://www.tga.gov.au/resources/prescription-medicines-registrations/nurtec-odt-pfizer-australia-pty-ltd.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Nurtec ODT - rimegepant sulfate tablet, orally disintegrating". 19 February 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9ef08e09-1098-35cc-e053-2a95a90a3e1d.
- ↑ 4.0 4.1 4.2 "Vydura EPAR". 22 February 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/vydura. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 5.0 5.1 5.2 "Drug Trials Snapshots: Nurtec ODT". 27 February 2020. https://www.fda.gov/drugs/development-approval-process-drugs/drug-trials-snapshots-nurtec-odt.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 6.0 6.1 "New therapeutic approaches for the prevention and treatment of migraine". The Lancet. Neurology 14 (10): 1010–1022. October 2015. doi:10.1016/S1474-4422(15)00198-2. PMID 26376968.
- ↑ 7.0 7.1 "Drug Approval Package: Nurtec ODT". 26 March 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212728Orig1s000TOC.cfm.
- ↑ "Pfizer Completes Acquisition of Biohaven Pharmaceuticals". Pfizer (Press release). 3 October 2022. Archived from the original on 5 October 2022. Retrieved 16 October 2022.
- ↑ "Biohaven Pharma's Nurtec ODT Approved In Israel For Acute Treatment Of Migraine". https://www.streetinsider.com/Corporate+News/Biohaven+Pharmas+%28BHVN%29+NURTEC+ODT+Approved+In+Israel+For+Acute+Treatment+Of+Migraine/18106014.html.
- ↑ "Nurtec ODT: Treatment and Prevention of Migraine? And How to Get It.". 14 April 2021. https://www.migraineagain.com/all-about-nurtec-odt-rimegepant/.
- ↑ "Biohaven's Nurtec ODT Approved In United Arab Emirates For Acute Treatment Of Migraine". Biohaven Pharmaceuticals (Press release). Archived from the original on 11 March 2021. Retrieved 3 June 2021.
- ↑ "Biohaven's Nurtec ODT (rimegepant) Receives FDA Approval for the Acute Treatment of Migraine in Adults" (Press release). Biohaven Pharmaceuticals Holding Company Ltd. 27 February 2020. Archived from the original on 26 July 2021. Retrieved 28 February 2020 – via PR Newswire.
- ↑ "Gepants for Acute and Preventive Migraine Treatment: A Narrative Review". Brain Sciences 12 (12): 1612. November 2022. doi:10.3390/brainsci12121612. PMID 36552072.
- ↑ 14.0 14.1 "Vydura: Pending EC decision". 24 February 2022. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/vydura. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Vydura Product information". https://ec.europa.eu/health/documents/community-register/html/h1645.htm.
External links
- Clinical trial number NCT03461757 for "Trial in Adult Subjects With Acute Migraines" at ClinicalTrials.gov
- Clinical trial number NCT03732638 for "Efficacy and Safety Trial of Rimegepant for Migraine Prevention in Adults" at ClinicalTrials.gov
 |

