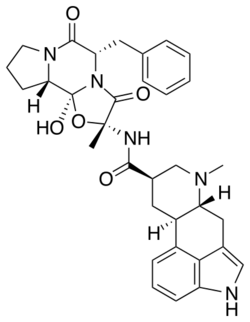
Chemistry:Dihydroergotamine
 | |
| Clinical data | |
|---|---|
| Pronunciation | /daɪˌhaɪdroʊ.ɜːrˈɡɒtəmiːn/ dy-HY-droh-ur-GOT-ə-meen |
| Trade names | D.H.E. 45, Migranal, Trudhesa, others |
| Other names | DHE; (5'α)-9,10-Dihydro-12'-hydroxy-2'-methyl-5'-(phenylmethyl)-ergotaman-3',6',18-trione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603022 |
| License data |
|
| Routes of administration | Nasal, Subcutaneous, Intramuscular, Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 32% (nasal spray) |
| Elimination half-life | 9 hours |
| Excretion | Bile |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| Chemical and physical data | |
| Formula | C33H37N5O5 |
| Molar mass | 583.689 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Dihydroergotamine (DHE), sold under the brand names D.H.E. 45 and Migranal among others, is an ergot alkaloid used to treat migraines.[5] It is a derivative of ergotamine. It is administered as a nasal spray or injection and has an efficacy similar to that of sumatriptan. Nausea is a common side effect.[6]
It has similar actions to the triptans, acting as an agonist to the serotonin receptors and causing vasoconstriction of the intracranial blood vessels, but also interacts centrally with dopamine and adrenergic receptors. It can be used to treat acute intractable headache or withdrawal from analgesics.
Medical uses
Subcutaneous and intramuscular injections are generally more effective than the nasal spray and can be self-administered by patients.[6] Intravenous injection is considered very effective for severe migraine or status migrainosus. DHE is also used in the treatment of medication overuse headache.[7]
Side effects
Nausea is a common side effect of IV administration and less common in other modes.[citation needed] Antiemetics can be given prior to DHE to counteract the nausea. Risks and contraindications are similar to the triptans. DHE and triptans should never be taken within 24 hours of each other due to the potential for coronary artery vasospasm.[citation needed] DHE produces no dependence.[8]
Contraindications
DHE is contraindicated with potent CYP3A4 inhibitors, like macrolide antibiotics.[9]
Pharmacology
Mechanism of action
DHE's antimigraine activity is due to its action as an agonist at the serotonin 5-HT1B, 5-HT1D, and 5-HT1F receptors. It also interacts with other serotonin, adrenergic, and dopamine receptors.[10]
DHE is an agonist of the serotonin 5-HT2B receptor and has been associated with cardiac valvulopathy.[11]
Pharmacodynamic
| Site | Affinity (Ki/IC50 [nM]) | Efficacy (Emax [%]) | Action |
|---|---|---|---|
| 5-HT1A | 0.4–1.5 | ? | Agonist |
| 5-HT1B | 0.006–18 | ? | Agonist |
| 5-HT1D | 0.13–0.5 | ? | Agonist |
| 5-HT1E | 1,100 | ? | ? |
| 5-HT1F | 180 | ? | Agonist |
| 5-HT2A | 9.0 | ? | Agonist |
| 5-HT2B | 15–33 | ? | Agonist |
| 5-HT2C | 1.3 | ? | Agonist |
| 5-HT3 | >3,700–>10,000 | ? | ? |
| 5-HT4 | 60 | ? | ? |
| 5-HT5A | ? | ? | ? |
| 5-HT5B | ? | ? | |
| 5-HT6 | 5.4 | ? | ? |
| 5-HT7 | 9.1–9.2 | ? | ? |
| α1A | 6.6 | ? | ? |
| α1B | 8.3 | ? | ? |
| α1D | ? | ? | ? |
| α2A | 1.9 | ? | ? |
| α2B | 3.3 | ? | ? |
| α2C | 1.4 | ? | ? |
| β1 | 3,100 | ? | ? |
| β2 | 2,700 | ? | ? |
| β3 | 271 | ? | ? |
| D1 | 2,779 | ? | ? |
| D2 | 1.2–5.0 | ? | Agonist |
| D3 | 6.4–16 | ? | ? |
| D4 | 8.7 | ? | ? |
| D5 | ? | ? | ? |
| H1 | ? | ? | ? |
| mACh | ? | ? | ? |
| Notes: All receptors are human except 5-HT3 (rat/mouse), 5-HT4 (guinea pig), 5-HT5B (rat—no human counterpart), α1A-adrenergic (rat/human), and α2A-adrenergic (rat/human).[12] | |||
Pharmacokinetics
Oral bioavailability is poor and it is not available in oral form in the US. DHE is available as a nasal spray and in ampules for subcutaneous, intramuscular and intravenous injection. Efficacy is variable in the nasal spray form with relative bioavailability of 32% compared to injection.[citation needed]
Contraindications
Contraindications for DHE include: pregnancy, renal or hepatic failure, coronary, cerebral, and peripheral vascular disease, hypersensitivity reactions, sepsis, and uncontrolled hypertension.[9]
History
Dihydroergotamine (DHE) is a semi-synthetic form of ergotamine approved in the US in 1946.[14]
Society and culture
Brand names
Brand names of DHE include Diergo, Dihydergot, D.H.E. 45, Ergont, Ikaran, Migranal, Orstanorm, and Seglor, among others.[5]
European Union
In 2013 the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that medicines containing ergot derivatives no longer be used to treat several conditions involving problems with memory, sensation or blood circulation, or to prevent migraine headaches because the risks (increased risk of fibrosis and ergotism) were said to be greater than the benefits in these indications.[15]
References
- ↑ "D.H.E. 45- dihydroergotamine mesylate injection, solution". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fe826e6a-75c8-43ed-9c36-f8263ec35aff.
- ↑ "Migranal- dihydroergotamine mesylate spray". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a24befa8-b952-48ac-942a-379585250782.
- ↑ "Dromelate- dihydroergotamine mesylate injection, solution". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2367e853-1109-4c23-a177-6829304832e8.
- ↑ "Trudhesa- dihydroergotamine mesylate spray, metered". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b81397b0-910d-4eb4-8fee-a9a3c9d94d63.
- ↑ 5.0 5.1 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 340–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA340.
- ↑ 6.0 6.1 "Parenteral dihydroergotamine for acute migraine headache: a systematic review of the literature". Annals of Emergency Medicine 45 (4): 393–401. April 2005. doi:10.1016/j.annemergmed.2004.07.430. PMID 15795718.
- ↑ "DHE in the pharmacotherapy of migraine: potential for a larger role". Headache 46 (Suppl 4): S212–S220. November 2006. doi:10.1111/j.1526-4610.2006.00605.x. PMID 17078853.
- ↑ 8.0 8.1 "Agonist actions of dihydroergotamine at 5-HT2B and 5-HT2C receptors and their possible relevance to antimigraine efficacy". British Journal of Pharmacology 140 (2): 277–284. September 2003. doi:10.1038/sj.bjp.0705437. PMID 12970106.
- ↑ 9.0 9.1 "Ergotamine and dihydroergotamine: a review". Current Pain and Headache Reports 7 (1): 55–62. February 2003. doi:10.1007/s11916-003-0011-7. PMID 12525272.
- ↑ "Ergotamine and dihydroergotamine: history, pharmacology, and efficacy". Headache 43 (2): 144–166. February 2003. doi:10.1046/j.1526-4610.2003.03034.x. PMID 12558771.
- ↑ "Safety Pharmacology assessment of drugs with biased 5-HT(2B) receptor agonism mediating cardiac valvulopathy". Journal of Pharmacological and Toxicological Methods 69 (2): 150–161. 2014. doi:10.1016/j.vascn.2013.12.004. PMID 24361689.
- ↑ 12.0 12.1 "Ergotamine search results". PDSP Ki Database. University of North Carolina Chapel Hill. https://pdsp.unc.edu/databases/pdsp.php?testFreeRadio=testFreeRadio&testLigand=Ergotamine&doQuery=Submit+Query.
- ↑ "Ergotamine and dihydroergotamine: history, pharmacology, and efficacy". Headache 43 (2): 144–166. February 2003. doi:10.1046/j.1526-4610.2003.03034.x. PMID 12558771.
- ↑ "Dihydroergotamine (DHE) - Then and Now: A Narrative Review". Headache 60 (1): 40–57. January 2020. doi:10.1111/head.13700. PMID 31737909.
- ↑ Restrictions on use of medicines containing ergot derivatives (EMA 2013) , Retrieved 3 August 2014
External links
- "Dihydroergotamine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/dihydroergotamine.
- "Dihydroergotamine mesylate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/dihydroergotamine%20mesylate.
 |
