Chemistry:Ubrogepant
 | |
| Clinical data | |
|---|---|
| Trade names | Ubrelvy |
| Other names | MK-1602 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620016 |
| License data |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 87% (in vitro) |
| Elimination half-life | 5-7 hrs |
| Excretion | fecal/biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
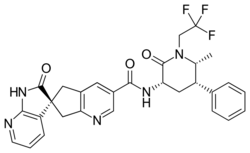
| Formula | C29H26F3N5O3 |
| Molar mass | 549.554 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ubrogepant, sold under the brand name Ubrelvy, is a medication used for the acute (immediate) treatment of migraine with or without aura (a sensory phenomenon or visual disturbance) in adults.[3][4] It is not indicated for the preventive treatment of migraine.[5] Ubrogepant is a small-molecule calcitonin gene-related peptide receptor antagonist.[6][7] It is the first drug in this class approved for the acute treatment of migraine.[8]
The most common side effects are nausea, tiredness and dry mouth.[5] Ubrogepant is contraindicated for co-administration with strong CYP3A4 inhibitors.[5]
History
Ubrogepant, also known as MK-1602, was discovered by scientists at Merck.[9]
The effectiveness of ubrogepant for the acute treatment of migraine was demonstrated in two randomized, double-blind, placebo-controlled trials.[5] In these studies, 1,439 adult patients with a history of migraine, with and without aura, received the approved doses of ubrogepant to treat an ongoing migraine.[5] In both studies, the percentages of patients achieving pain relief two hours after treatment (defined as a reduction in headache severity from moderate or severe pain to no pain) and whose most bothersome migraine symptom (nausea, light sensitivity or sound sensitivity) stopped two hours after treatment were significantly greater among patients receiving ubrogepant (19–21% depending on the dose) compared to those receiving placebo (12%).[5][10] Patients were allowed to take their usual acute treatment of migraine at least two hours after taking ubrogepant.[5] 23% of patients were taking a preventive medication for migraine.[5]
In December 2019, the U.S. Food and Drug Administration approved Ubrelvy produced by Allergan USA, Inc. for treatment of migraine after onset.[5][11]
References
- ↑ "Ubrelvy Product information". https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=102156.
- ↑ "Summary Basis of Decision - Ubrelvy". 30 March 2023. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00631&lang=en.
- ↑ 3.0 3.1 "Ubrelvy- ubrogepant tablet". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fd9f9458-fd96-4688-be3f-f77b3d1af6ab.
- ↑ "Ubrogepant Prescribing Information". U.S. Food and Drug Administration. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211765s000lbl.pdf.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "FDA approves new treatment for adults with migraine". U.S. Food and Drug Administration (FDA) (Press release). 23 December 2019. Archived from the original on 23 December 2019. Retrieved 23 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Possible site of action of CGRP antagonists in migraine". Cephalalgia 31 (6): 748–750. April 2011. doi:10.1177/0333102411398403. PMID 21383046.
- ↑ "Neue Wirkstoffe: Ubrogepant" (in German). Österreichische Apotheker-Zeitung (11/2018). 22 May 2018.
- ↑ "What You Need to Know About Ubrelvy, First Acute CGRP Med Approved" (in en-US). 2020-01-28. https://www.migraineagain.com/first-oral-cgrp-for-migraine-ubrelvy-is-approved-by-fda/.
- ↑ "Characterization of Ubrogepant: A Potent and Selective Antagonist of the Human Calcitonin Gene‒Related Peptide Receptor". The Journal of Pharmacology and Experimental Therapeutics: jpet.119.261065. January 2020. doi:10.1124/jpet.119.261065. PMID 31992609.
- ↑ "Ubrogepant for the Treatment of Migraine". The New England Journal of Medicine 381 (23): 2230–2241. December 2019. doi:10.1056/NEJMoa1813049. PMID 31800988.
- ↑ "Allergan's acute migraine treatment wins U.S. FDA approval". Reuters. 23 December 2019. https://www.reuters.com/article/us-allergan-fda/allergans-acute-migraine-treatment-wins-u-s-fda-approval-idUSKBN1YR1Y9.
External links
- "Ubrogepant". Drug Information Portal. United States National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/ubrogepant.
- "Drug Trials Snapshots: Ubrelvy". U.S. Food and Drug Administration (FDA). 13 January 2020. http://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-ubrelvy.
 |

