Chemistry:Scytonemin
| |
| Names | |
|---|---|
| Preferred IUPAC name
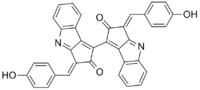
(3E,3′E)-3,3′-Bis[(4-hydroxyphenyl)methylidene][1,1′-bi(cyclopropa[b]indole)]-2,2′(3H,3′H)-dione | |
| Other names
Scytonemin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C36H20N2O4 | |
| Molar mass | 544.6 g/mol |
| Appearance | brown solid |
| Solubility | 25mg/ml DMSO |
| UV-vis (λmax) | 370nm |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Scytonemin is a secondary metabolite and an extracellular matrix (sheath) pigment synthesized by many strains of cyanobacteria, including Nostoc, Scytonema, Calothrix, Lyngbya, Rivularia, Chlorogloeopsis, and Hyella.[1] Scytonemin-synthesizing cyanobacteria often inhabit highly insolated terrestrial, freshwater and coastal environments such as deserts, semideserts, rocks, cliffs, marine intertidal flats, and hot springs.[2]
The pigment was originally discovered in 1849 by Swiss botanist Carl Nägeli,[3] although the structure remained unsolved until 1993.[4] It is an aromatic indole alkaloid built from two identical condensation products of tryptophanyl- and tyrosyl-derived subunits linked through a carbon-carbon bond.[4] Depending on the redox conditions it can exist in two inter-convertible forms: a more common oxidized yellow-brown form which is insoluble in water and only slightly soluble in organic solvents, such as pyridine, and a reduced form with bright red color that is more soluble in organic solvents.[5] Scytonemin absorbs very strongly and very broadly across the UV-C-UV-B-UV-A-violet-blue spectral region, with an in vivo maximum absorption at 370 nm and an in vitro maximum absorption at 386 and 252 nm, and with smaller peaks at 212, 278 and 300 nm.[6]
It is believed that scytonemin acts as a highly efficient protective biomolecule (sunscreen) that filters out damaging high frequency UV rays while at the same time allowing the transmittance of wavelengths necessary for photosynthesis.[7] Its biosynthesis in cyanobacteria is mostly triggered by exposure to UV-A and UV-B wavelengths.[8][9]
Recently, Couradeau and coworkers found that cyanobacterial soil crusts warm the soil surface by as much as 10 °C through the production and accumulation of scytonemin pigments.[10] This effect is due to the dissipation of the absorbed photons by the scytonemin molecules into heat.
Biosynthesis
The biosynthesis in Lyngbya aestuarii was recently explored by Balskus, Case, and Walsh. It proceeds by the conversion of L-tryptophan to 3-indole pyruvic acid, followed by coupling to p-hydroxyphenylpyruvic acid. Cyclization of the resultant β-ketoacid yields a tricyclic ketone. Oxidation and dimerization yields the completed natural product. Three scytonemin biosynthetic enzymes are necessary, denoted as ScyA-C.[11]
References
- ↑ Sinha, Hader (2008-03-01). "UV-protectants in cyanobacteria". Plant Science 174 (3): 278–289. doi:10.1016/j.plantsci.2007.12.004. ISSN 0168-9452.
- ↑ (in en) Ecology of Cyanobacteria II - Their Diversity in Space and | Brian A. Whitton | Springer. Springer. 2012. ISBN 9789400738546. https://www.springer.com/us/book/9789400738546.
- ↑ Nägeli, Carl (1849). Gattungen einzelliger Algen physiologisch und systematisch bearbeitet. MBLWHOI Library. Zürich, Friedrich Schulthess. https://archive.org/details/gattungeneinzell00ng.
- ↑ 4.0 4.1 Proteau, P. J.; Gerwick, W. H.; Garcia-Pichel, F.; Castenholz, R. (1993). "The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria". Experientia 49 (9): 825–9. doi:10.1007/BF01923559. PMID 8405307.
- ↑ Garcia-Pichel, Ferran; Castenholz, Richard W. (1991-06-01). "Characterization and Biological Implications of Scytonemin, a Cyanobacterial Sheath Pigment1" (in en). Journal of Phycology 27 (3): 395–409. doi:10.1111/j.0022-3646.1991.00395.x. ISSN 1529-8817.
- ↑ Sinha, Rajeshwar; Klisch, M; Vaishampayan, Akhouri; Häder, Donat (1999-11-01). "Biochemical and spectroscopic characterization of the cyanobacterium Lyngbya sp. inhabiting mango (Mangifera indica) trees: Presence of an ultraviolet-absorbing pigment, scytonemin". Acta Protozoologica 38: 291–298. https://www.researchgate.net/publication/266383468.
- ↑ Ekebergh, Andreas; Sandin, Peter; Mårtensson, Jerker (2015-11-25). "On the photostability of scytonemin, analogues thereof and their monomeric counterparts" (in en). Photochemical & Photobiological Sciences 14 (12): 2179–2186. doi:10.1039/C5PP00215J. ISSN 1474-9092. PMID 26452010.
- ↑ Sorrels, Carla M.; Proteau, Philip J.; Gerwick, William H. (2009-07-15). "Organization, Evolution, and Expression Analysis of the Biosynthetic Gene Cluster for Scytonemin, a Cyanobacterial UV-Absorbing Pigment" (in en). Applied and Environmental Microbiology 75 (14): 4861–4869. doi:10.1128/AEM.02508-08. ISSN 0099-2240. PMID 19482954.
- ↑ Rastogi, Rajesh P.; Incharoensakdi, Aran (2014-01-01). "Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacteriumLyngbyasp. CU2555" (in en). FEMS Microbiology Ecology 87 (1): 244–256. doi:10.1111/1574-6941.12220. ISSN 0168-6496. PMID 24111939.
- ↑ Couradeau, Estelle; Karaoz, Ulas; Lim, Hsiao Chien; Rocha, Ulisses Nunes da; Northen, Trent; Brodie, Eoin; Garcia-Pichel, Ferran (2016-01-20). "Bacteria increase arid-land soil surface temperature through the production of sunscreens" (in En). Nature Communications 7: 10373. doi:10.1038/ncomms10373. PMID 26785770. Bibcode: 2016NatCo...710373C.
- ↑ 11.0 11.1 Balskus, Emily P.; Case, Rebecca J.; Walsh, Christopher T. (2011). "The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities". FEMS Microbiology Ecology 77 (2): 1–11. doi:10.1111/j.1574-6941.2011.01113.x. PMID 21501195. PMC 3134115. https://dash.harvard.edu/bitstream/handle/1/12169551/nihms292647.pdf?sequence=1.
 |
![Scytonemin biosynthesis in Lyngbya aestuarii.[11]](/wiki/images/thumb/3/30/Scytonemin_biosynthesis.png/880px-Scytonemin_biosynthesis.png)

