Chemistry:Mandelic acid
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hydroxy(phenyl)acetic acid | |||
| Other names
2-Hydroxy-2-phenylacetic acid
Mandelic acid Phenylglycolic acid α-Hydroxyphenylacetic acid | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C8H8O3 | |||
| Molar mass | 152.149 g·mol−1 | ||
| Appearance | White crystalline powder | ||
| Density | 1.30 g/cm3 | ||
| Melting point | 119 °C (246 °F; 392 K) optically pure: 132 to 135 °C (270 to 275 °F; 405 to 408 K) | ||
| Boiling point | 321.8 °C (611.2 °F; 595.0 K) | ||
| 15.87 g/100 mL | |||
| Solubility | soluble in diethyl ether, ethanol, isopropanol | ||
| Acidity (pKa) | 3.41[2] | ||
Refractive index (nD)
|
1.5204 | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
0.1761 kJ/g | ||
| Pharmacology | |||
| 1=ATC code }} | B05CA06 (WHO) J01XX06 (WHO) | ||
| Hazards | |||
| Flash point | 162.6 °C (324.7 °F; 435.8 K) | ||
| Related compounds | |||
Related compounds
|
mandelonitrile, phenylacetic acid, vanillylmandelic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as paramandelic acid.
Isolation, synthesis, occurrence
Mandelic acid was discovered in 1831 by the German pharmacist Ferdinand Ludwig Winckler (1801–1868) while heating amygdalin, an extract of bitter almonds, with diluted hydrochloric acid. The name is derived from the German "Mandel" for "almond".[3]
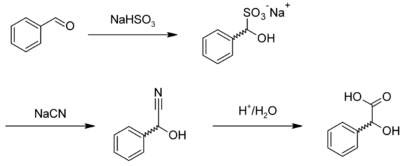
Mandelic acid is usually prepared by the acid-catalysed hydrolysis of mandelonitrile,[4] which is the cyanohydrin of benzaldehyde. Mandelonitrile can also be prepared by reacting benzaldehyde with sodium bisulfite to give the corresponding adduct, forming mandelonitrile with sodium cyanide, which is hydrolyzed:[5]
Alternatively, it can be prepared by base hydrolysis of phenylchloroacetic acid as well as dibromacetophenone.[6] It also arises by heating phenylglyoxal with alkalis.[7][8]
Biosynthesis
Mandelic acid is a substrate or product of several biochemical processes called the mandelate pathway. Mandelate racemase interconverts the two enantiomers via a pathway that involves cleavage of the alpha-CH bond. Mandelate dehydrogenase is yet another enzyme on this pathway.[9] Mandelate also arises from trans-cinnamate via phenylacetic acid, which is hydroxylated.[10] Phenylpyruvic acid is another precursor to mandelic acid.
Derivatives of mandelic acid are formed as a result of metabolism of adrenaline and noradrenaline by monoamine oxidase and catechol-O-methyl transferase. The biotechnological production of 4-hydroxy-mandelic acid and mandelic acid on the basis of glucose was demonstrated with a genetically modified yeast Saccharomyces cerevisiae, in which the hydroxymandelate synthase naturally occurring in the bacterium Amycolatopsis was incorporated into a wild-type strain of yeast, partially altered by the exchange of a gene sequence and expressed.[11]
It also arises from the biodegradation of styrene [12] and ethylbenzene, as detected in urine.
Uses
Mandelic acid has a long history of use in the medical community as an antibacterial, particularly in the treatment of urinary tract infections.[13] It has also been used as an oral antibiotic, and as a component of chemical face peels analogous to other alpha hydroxy acids.[14]
The drugs cyclandelate and homatropine are esters of mandelic acid.
References
- ↑ Merck Index, 11th Edition, 5599.
- ↑ Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- ↑ See:
- Winckler, F. L. (1831) "Ueber die Zersetzung des Calomels durch Bittermandelwasser, und einige Beiträge zur genaueren Kenntniss der chemischen Zusammensetzung des Bittermandelwassers" (On the decomposition of calomel [i.e., mercury(I) chloride] by bitter almond water, and some contributions to a more precise knowledge of the chemical composition of bitter almond water), Repertorium für die Pharmacie, 37 : 388–418; mandelic acid is named on p. 415.
- Winckler, F. L. (1831) "Ueber die chemische Zusammensetzung des Bittermandelwassers; als Fortsetzung der im 37sten Band S. 388 u.s.w. des Repertoriums enthaltenen Mittheilungen" [On the chemical composition of bitter almond water; as a continuation of the report contained in the 37th volume, pp. 388 ff. of the Repertorium], Repertorium für die Pharmacie, 38 : 169–196. On p. 193, Winckler describes the preparation of mandelic acid from bitter almond water and hydrochloric acid (Salzsäure).
- (Editor) (1832) "Ueber einige Bestandtheile der Bittermandeln" (On some components of bitter almonds), Annalen der Chemie und Pharmacie, 4 : 242–247.
- Winckler, F. L. (1836) "Ueber die Mandelsäure und einige Salze derselben" (On mandelic acid and some salts of the same), Annalen der Chemie und Pharmacie, 18 (3) : 310–319.
- Hermann Schelenz, Geschichte der Pharmazie [The History of Pharmacy] (Berlin, German: Julius Springer, 1904), p. 675.
- ↑ Ritzer, Edwin; Sundermann, Rudolf (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_519.
- ↑ Corson, B. B.; Dodge, R. A.; Harris, S. A.; Yeaw, J. S. (1926). "Mandelic Acid". Org. Synth. 6: 58. doi:10.15227/orgsyn.006.0058.
- ↑ J. G. Aston; J. D. Newkirk; D. M. Jenkins; Julian Dorsky (1952). "Mandelic Acid". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv3p0538.; Collective Volume, 3, pp. 538
- ↑ Pechmann, H. von (1887). "Zur Spaltung der Isonitrosoverbindungen". Berichte der Deutschen Chemischen Gesellschaft 20 (2): 2904–2906. doi:10.1002/cber.188702002156. https://zenodo.org/record/1425483.
- ↑ Pechmann, H. von; Muller, Hermann (1889). "Ueber α-Ketoaldehyde". Berichte der Deutschen Chemischen Gesellschaft 22 (2): 2556–2561. doi:10.1002/cber.188902202145. https://zenodo.org/record/1425555.
- ↑ Kenyon, George L.; Gerlt, John A.; Petsko, Gregory A.; Kozarich, John W. (1995). "Mandelate Racemase: Structure-Function Studies of a Pseudosymmetric Enzyme". Accounts of Chemical Research 28 (4): 178–186. doi:10.1021/ar00052a003.
- ↑ Lapadatescu, Carmen; Giniès, Christian; Le QuéRé, Jean-Luc; Bonnarme, Pascal (2000). "Novel Scheme for Biosynthesis of Aryl Metabolites from l-Phenylalanine in the Fungus Bjerkandera adusta". Applied and Environmental Microbiology 66 (4): 1517–1522. doi:10.1128/AEM.66.4.1517-1522.2000. PMID 10742235. Bibcode: 2000ApEnM..66.1517L.
- ↑ Mara Reifenrath, Eckhard Boles: Engineering of hydroxymandelate synthases and the aromatic amino acid pathway enables de novo biosynthesis of mandelic and 4-hydroxymandelic acid with Saccharomyces cerevisiae. Metabolic Engineering 45, Januar 2018; S. 246-254. doi:10.1016/j.ymben.2018.01.001.
- ↑ Engström K, Härkönen H, Kalliokoski P, Rantanen J. "Urinary mandelic acid concentration after occupational exposure to styrene and its use as a biological exposure test" Scand. J. Work Environ. Health. 1976, volume 2, pp. 21-6.
- ↑ Putten, P. L. (1979). "Mandelic acid and urinary tract infections". Antonie van Leeuwenhoek 45 (4): 622–623. doi:10.1007/BF00403669.
- ↑ Taylor, MB. (1999). "Summary of mandelic acid for the improvement of skin conditions". Cosmetic Dermatology 21: 26–28.
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Mandelic Acid". Encyclopædia Britannica. 17 (11th ed.). Cambridge University Press. p. 559.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Mandelic Acid". Encyclopædia Britannica. 17 (11th ed.). Cambridge University Press. p. 559.