Chemistry:Tert-Butyl chloride
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Chloro-2-methylpropane | |||
| Other names
1,1-dimethylethyl chloride
1-chloro-1,1-dimethylethane chlorotrimethylmethane trimethylchloromethane t-butyl chloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1127 | ||
| |||
| |||
| Properties | |||
| C4H9Cl | |||
| Molar mass | 92.57 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.851 g/ml | ||
| Melting point | −26 °C (−15 °F; 247 K) | ||
| Boiling point | 51 °C (124 °F; 324 K) | ||
| Sparingly soluble in water, miscible with alcohol and ether | |||
| Vapor pressure | 34.9 kPa (20 °C) | ||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Danger | ||
| H225 | |||
| P210, P233, P240, P241, P242, P243, P280, P303+361+353, P370+378, P403+235, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −9 °C (16 °F; 264 K) (open cup) −23 °C (closed cup) | ||
| 540 °C (1,004 °F; 813 K) | |||
| Related compounds | |||
Related alkyl halides
|
tert-Butyl bromide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
tert-Butyl chloride is the organochloride with the formula (CH
3)
3CCl. It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol. It is produced industrially as a precursor to other organic compounds.[1]
Synthesis
tert-Butyl chloride is produced by the reaction of tert-butyl alcohol with hydrogen chloride.[1] In the laboratory, concentrated hydrochloric acid is used. The conversion entails a SN1 reaction as shown below.[2]
| Step 1 | Step 2 | Step 3 |
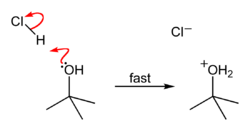 |
250px | 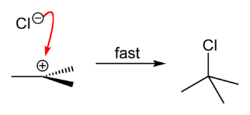
|
| The acid protonates the alcohol, forming a good leaving group (water). | Water leaves the protonated t-BuOH, forming a relatively stable tertiary carbocation. | The chloride ion attacks the carbocation, forming t-BuCl. |
The overall reaction, therefore, is:
- (CH
3)
3COH + HCl → (CH
3)
3CCl + H
2O
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.
Reactions
When tert-butyl chloride is dissolved in water, it undergoes a hydrolysis to tert-butyl alcohol. When dissolved in alcohols, the corresponding t-butyl ethers are produced.
Uses
tert-Butyl chloride is used to prepare the antioxidant tert-butylphenol and the fragrance neohexyl chloride.[1]
See also
References
- ↑ 1.0 1.1 1.2 M. Rossberg et al. "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ↑ James F. Norris and Alanson W. Olmsted "tert-Butyl Chloride" Org. Synth. 1928, volume 8, pp. 50. doi:10.15227/orgsyn.008.0050
External links
- Safety MSDS data
- Preparation 2-chloro-2-methylpropane
- http://www.cerlabs.com/experiments/10875407331.pdf
 |