Chemistry:Tris(acetylacetonato)titanium(III)
| |
| Names | |
|---|---|
| IUPAC name
4-oxopent-2-en-2-olate;titanium(3+)
| |
| Other names
tris(2,4-pentanedionato)titanium, titanium trisacetylacetonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C15H21O6Ti | |
| Molar mass | 345.194 g·mol−1 |
| Appearance | blue solid |
| Density | 1.366 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
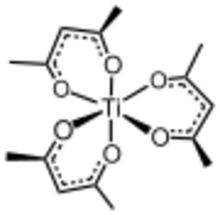
Tris(acetylacetonato)titanium(III), often abbreviated Ti(acac)3, is a coordination complex of titanium(III) featuring acetylacetonate (acac) ligands, making it one of a family of metal acetylacetonates. It is a blue air-sensitive solid that dissolves in nonpolar organic solvents. The compound is prepared by treating titanium trichloride with acetylacetone in the presence of base.[1] Being paramagnetic, it gives a contact-shifted proton NMR signal at 60 ppm upfield of TMS assigned to the methyl group.[2]
Structure
It is an octahedral complex. The Ti-O bonds lengths range from 2.023 to 2.013 Å, the large variation being attributed to the Jahn-Teller effect. Ti(acac)3 possesses helical chirality, giving rise to Δ- and Λ-enantiomers.
It is a precatalyst for Ziegler-Natta catalysis.[3]
References
- ↑ Arslan, Evrim; Lalancette, Roger A.; Bernal, Ivan (2017). "An Historic and Scientific Study of the Properties of Metal(III) Tris-acetylacetonates". Structural Chemistry 28: 201–212. doi:10.1007/s11224-016-0864-0.
- ↑ Eaton, D. R. (1965). "The Nuclear Magnetic Resonance of Some Paramagnetic Transition Metal Acetylacetonates". Journal of the American Chemical Society 87 (14): 3097–3102. doi:10.1021/ja01092a015.
- ↑ Zambelli, Adolfo; Oliva, Leone; Pellecchia, Claudio (1989). "Soluble Catalysts for Syndiotactic Polymerization of Styrene". Macromolecules 22 (5): 2129–2130. doi:10.1021/ma00195a021. Bibcode: 1989MaMol..22.2129Z.
 |

