Chemistry:Titanium hydride
 Titanium hydride powder
| |
| Names | |
|---|---|
| IUPAC name
titanium dihydride (hydrogen deficient)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1871 (TITANIUM HYDRIDE) |
| |
| |
| Properties | |
| TiH 2-x | |
| Molar mass | 49.88 g/mol (TiH 2) |
| Appearance | black powder (commercial form) |
| Density | 3.76 g/cm3 (typical commercial form) |
| Melting point | Decomposes |
| insoluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H228 | |
| P210, P240, P241, P280, P370+378 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Titanium hydride normally refers to the inorganic compound TiH
2 and related nonstoichiometric materials.[1][2] It is commercially available as a stable grey/black powder, which is used as an additive in the production of Alnico sintered magnets, in the sintering of powdered metals, the production of metal foam, the production of powdered titanium metal and in pyrotechnics.[3]
Also known as titanium–hydrogen alloy,[4][5] it is an alloy[6] of titanium, hydrogen, and possibly other elements. When hydrogen is the main alloying element, its content in the titanium hydride is between 0.02% and 4.0% by weight. Alloying elements intentionally added to modify the characteristics of titanium hydride include gallium, iron, vanadium, and aluminium.
Production
In the commercial process for producing non-stoichiometric TiH
2-x, titanium metal sponge is treated with hydrogen gas at atmospheric pressure at between 300-500 °C. Absorption of hydrogen is exothermic and rapid, changing the color of the sponge grey/black. The brittle product is ground to a powder, which has a composition around TiH
1.95.[3] In the laboratory, titanium hydride is produced by heating titanium powder under flowing hydrogen at 700 °C, the idealized equation being:[7]
- Ti + H
2 → TiH
2
Other methods of producing titanium hydride include electrochemical and ball milling methods.[8][9]
Reactions
TiH
1.95 is unaffected by water and air. It is slowly attacked by strong acids and is degraded by hydrofluoric and hot sulfuric acids. It reacts rapidly with oxidizing agents, this reactivity leading to the use of titanium hydride in pyrotechnics.[3]
The material has been used to produce highly pure hydrogen, which is released upon heating the solid.[7] Hydrogen release in TiH~2 starts just above 400 °C but may not be complete until the melting point of titanium metal.[10][3] Titanium tritide[broken anchor] (Ti3Hx) has been proposed for long-term storage of tritium gas.[11]
Structure
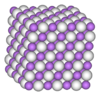
As TiH
x approaches stoichiometry, it adopts a distorted body-centered tetragonal structure, termed the ε-form with an axial ratio of less than 1. This composition is very unstable with respect to partial thermal decomposition, unless maintained under a pure hydrogen atmosphere. Otherwise, the composition rapidly decomposes at room temperature until an approximate composition of TiH
1.74 is reached. This composition adopts the fluorite structure, and is termed the δ-form, and only very slowly thermally decomposing at room temperature until an approximate composition of TiH
1.47 is reached, at which point, inclusions of the hexagonal close packed α-form, which is the same form as pure titanium, begin to appear.
The evolution of the dihydride from titanium metal and hydrogen has been examined in some detail. α-Titanium has a hexagonal close packed (hcp) structure at room temperature. Hydrogen initially occupies tetrahedral interstitial sites in the titanium. As the H/Ti ratio approaches 2, the material adopts the β-form to a face centred cubic (fcc), δ-form, the H atoms eventually filling all the tetrahedral sites to give the limiting stoichiometry of TiH
2. The various phases are described in the table below.
| Phase | Weight % H | Atomic % H | TiH x |
Metal lattice |
|---|---|---|---|---|
| α | 0 – 0.2 | 0 – 8 | TiH 0 – TiH 0.1 |
hcp |
| α & β | 0.2 – 1.1 | 8 – 34 | TiH 0.1 – TiH 0.5 |
|
| β | 1.1 – 1.8 | 34 – 47 | TiH 0.5 – TiH 0.9 |
bcc |
| β & δ | 1.8 – 2.5 | 47 – 57 | TiH 0.9 – TiH 1.32 |
|
| δ | 2.7 – 4.1 | 57 – 67 | TiH 1.32 – TiH 2 |
fcc |
If titanium hydride contains 4.0% hydrogen at less than around 40 °C then it transforms into a body-centred tetragonal (bct) structure called ε-titanium.[12]
When titanium hydrides with less than 1.3% hydrogen, known as hypoeutectoid titanium hydride are cooled, the β-titanium phase of the mixture attempts to revert to the α-titanium phase, resulting in an excess of hydrogen. One way for hydrogen to leave the β-titanium phase is for the titanium to partially transform into δ-titanium, leaving behind titanium that is low enough in hydrogen to take the form of α-titanium, resulting in an α-titanium matrix with δ-titanium inclusions.
A metastable γ-titanium hydride phase has been reported.[13] When α-titanium hydride with a hydrogen content of 0.02-0.06% is quenched rapidly, it forms into γ-titanium hydride, as the atoms "freeze" in place when the cell structure changes from hcp to fcc. γ-Titanium takes a body centred tetragonal (bct) structure. Moreover, there is no compositional change so the atoms generally retain their same neighbours.
Hydrogen embrittlement in titanium and titanium alloys

The absorption of hydrogen and the formation of titanium hydride are a source of damage to titanium and titanium alloys. This hydrogen embrittlement process is of particular concern when titanium and alloys are used as structural materials, as in nuclear reactors.
Hydrogen embrittlement manifests as a reduction in ductility and eventually spalling of titanium surfaces. The effect of hydrogen is to a large extent determined by the composition, metallurgical history and handling of the Ti and Ti alloy.[14] CP-titanium (commercially pure: ≤99.55% Ti content) is more susceptible to hydrogen attack than pure α-titanium. Embrittlement, observed as a reduction in ductility and caused by the formation of a solid solution of hydrogen, can occur in CP-titanium at concentrations as low as 30-40 ppm. Hydride formation has been linked to the presence of iron in the surface of a Ti alloy. Hydride particles are observed in specimens of Ti and Ti alloys that have been welded, and because of this welding is often carried out under an inert gas shield to reduce the possibility of hydride formation.[14]
Ti and Ti alloys form a surface oxide layer, composed of a mixture of Ti(II), Ti(III) and Ti(IV) oxides,[15] which offers a degree of protection to hydrogen entering the bulk.[14] The thickness of this can be increased by anodizing, a process which also results in a distinctive colouration of the material. Ti and Ti alloys are often used in hydrogen containing environments and in conditions where hydrogen is reduced electrolytically on the surface. Pickling, an acid bath treatment which is used to clean the surface can be a source of hydrogen.
Uses
Common applications include ceramics, pyrotechnics, sports equipment, as a laboratory reagent, as a blowing agent, and as a precursor to porous titanium. When heated as a mixture with other metals in powder metallurgy, titanium hydride releases hydrogen which serves to remove carbon and oxygen, producing a strong alloy.[3]
The density of titanium hydride varies based on the alloying constituents, but for pure titanium hydride it ranges between 3.76 and 4.51 g/cm3.
Even in the narrow range of concentrations that make up titanium hydride, mixtures of hydrogen and titanium can form a number of different structures, with very different properties. Understanding such properties is essential to making quality titanium hydride. At room temperature, the most stable form of titanium is the hexagonal close-packed (HCP) structure α-titanium. It is a fairly hard metal that can dissolve only a small concentration of hydrogen, no more than 0.20 wt% at 464 °C (867 °F), and only 0.02% at 25 °C (77 °F). If titanium hydride contains more than 0.20% hydrogen at titanium hydride-making temperatures it transforms into a body-centred cubic (BCC) structure called β-titanium. It can dissolve considerably more hydrogen, more than 2.1% hydrogen at 636 °C (1,177 °F). If titanium hydride contains more than 2.1% at 636 °C (1,177 °F) then it transforms into a face-centred cubic (FCC) structure called δ-titanium. It can dissolve even more hydrogen, as much as 4.0% hydrogen 37 °C (99 °F), which reflects the upper hydrogen content of titanium hydride.[16]
There are many types of heat treating processes available to titanium hydride. The most common are annealing and quenching. Annealing is the process of heating the titanium hydride to a sufficiently high temperature to soften it. This process occurs through three phases: recovery, recrystallization, and grain growth. The temperature required to anneal titanium hydride depends on the type of annealing. Annealing must be done under a hydrogen atmosphere to prevent outgassing.
See also
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Wiberg, Egon; Wiberg, Nils; Holleman, A. F. (2001). Inorganic chemistry (1st English ed.). San Diego : Berlin ; New York: Academic Press ; De Gruyter. ISBN 0-12-352651-5.
- ↑ 3.0 3.1 3.2 3.3 3.4 Rittmeyer, Peter; Weitelmann, Ulrich (2005). "Hydrides". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a13_199. ISBN 978-3-527-30673-2.
- ↑ McQuillan, A. D. (22 December 1950). "An experimental and thermodynamic investigation of the hydrogen-titanium system". Proceedings of the Royal Society A 204 (1078): 309–323. doi:10.1098/rspa.1950.0176. Bibcode: 1950RSPSA.204..309M. http://rspa.royalsocietypublishing.org/content/204/1078/309.abstract. Retrieved 10 March 2013.
- ↑ Bennett, L. H. (1980). "Nuclear magnetic resonance in alloys". MRS Proceedings 3. doi:10.1557/PROC-3-3. http://journals.cambridge.org/action/displayAbstract;jsessionid=7611C84A9BF6AE1B9B569DE4D6BE39FC.journals?fromPage=online&aid=8088647. Retrieved 10 March 2013.
- ↑ Wang, Xin-Quan; Wang, Jian-Tao (15 June 2010). "Structural stability and hydrogen diffusion in TiHx alloys". Solid State Communications 150 (35–36): 1715–1718. doi:10.1016/j.ssc.2010.06.004. Bibcode: 2010SSCom.150.1715W. http://www.sciencedirect.com/science/article/pii/S0038109810003285. Retrieved 10 March 2013.
- ↑ 7.0 7.1 M. Baudler "Hydrogen, Deuterium, Water" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 114-115.
- ↑ Millenbach, Pauline; Givon, Meir (1 October 1982). "The electrochemical formation of titanium hydride". Journal of the Less Common Metals 87 (2): 179–184. doi:10.1016/0022-5088(82)90086-8. https://dx.doi.org/10.1016/0022-5088%2882%2990086-8. Retrieved 10 March 2013.
- ↑ Zhang, Heng; Kisi, Erich H (1997). "Formation of titanium hydride at room temperature by ball milling". Journal of Physics: Condensed Matter 9 (11): L185–L190. doi:10.1088/0953-8984/9/11/005. ISSN 0953-8984. Bibcode: 1997JPCM....9L.185Z.
- ↑ Paulin, Irena; Donik, Črtomir; Mandrino, Djordje; Vončina, Maja; Jenko, Monika (January 2012). "Surface characterization of titanium hydride powder". Vacuum 86 (6): 608–613. doi:10.1016/j.vacuum.2011.07.054. Bibcode: 2012Vacuu..86..608P. https://linkinghub.elsevier.com/retrieve/pii/S0042207X11003010.
- ↑ Brown, Charles C.; Buxbaum, Robert E. (June 1988). "Kinetics of hydrogen absorption in alpha titanium". Metallurgical Transactions A 19 (6): 1425–1427. doi:10.1007/bf02674016. Bibcode: 1988MTA....19.1425B.
- ↑ 12.0 12.1 Fukai, Y (2005). The Metal-Hydrogen System, Basic Bulk Properties, 2d edition. Springer. ISBN 978-3-540-00494-3.
- ↑ Numakura, H; Koiwa, M; Asano, H; Izumi, F (1988). "Neutron diffraction study of the metastable γ titanium deuteride". Acta Metallurgica 36 (8): 2267–2273. doi:10.1016/0001-6160(88)90326-4. ISSN 0001-6160.
- ↑ 14.0 14.1 14.2 Donachie, Matthew J. (2000). Titanium: A Technical Guide. ASM International. ISBN 978-0-87170-686-7.
- ↑ Lu, Gang; Bernasek, Steven L.; Schwartz, Jeffrey (2000). "Oxidation of a polycrystalline titanium surface by oxygen and water". Surface Science 458 (1–3): 80–90. doi:10.1016/S0039-6028(00)00420-9. ISSN 0039-6028. Bibcode: 2000SurSc.458...80L.
- ↑ Setoyama, Daigo; Matsunaga, Junji; Muta, Hiroaki; Uno, Masayohi; Yamanaka, Shinsuke (3 November 2004). "Mechanical properties of titanium hydride". Journal of Alloys and Compounds 381 (1–2): 215–220. doi:10.1016/j.jallcom.2004.04.073.
External links
 |