Chemistry:Tungsten(VI) oxytetrafluoride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
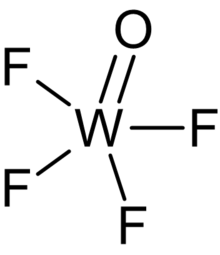
| WOF4 | |
| Molar mass | 275.83 g/mol |
| Appearance | colourless crystals[1] |
| Density | 5.07 g/cm3[2] |
| Melting point | 110[2] °C (230 °F; 383 K) |
| Boiling point | 185[2] °C (365 °F; 458 K) |
| reacts[2] | |
| Solubility | soluble in chloroform[3] sparingly soluble in carbon disulfide[3] |
| Structure | |
| monoclinic | |
| Related compounds | |
Other anions
|
Tungsten(VI) oxytetrachloride Tungsten(VI) oxytetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tungsten(VI) oxytetrafluoride (WOF4) is an inorganic chemical compound.
Preparation
Tungsten(VI) oxytetrafluoride can be synthesized by the reaction of fluorine and tungsten trioxide.[4]
It can also be obtained by reacting tungsten with a mixture of oxygen and fluorine at high temperatures.[1] Partial hydrolysis of tungsten hexafluoride will also produce WOF4.[5]
- [math]\ce{ WF6 + H2O -> WOF4 + 2 HF }[/math]
The reaction of tungsten(VI) oxytetrachloride and hydrogen fluoride will also produce WOF4.[3]
- [math]\ce{ WOCl4 + 4HF -> WOF4 + 4HCl }[/math]
WOF4 can also prepared by the reaction of lead(II) fluoride and tungsten trioxide at 700 °C.[3]
- [math]\ce{ 2PbF2 + WO3 -> WOF4 + 2PbO }[/math]
Properties
Tungsten(VI) oxytetrafluoride is a colourless solid which hydrolyze into tungstic acid.[1][5]
- [math]\ce{ WOF4 + 2 H2O -> WO3 + 4 HF }[/math]
Solid WOF4 is previously assumed as a tetramer,[6] which was later questioned by spectroscopic investigations. In the gas state, this molecule has a monomeric structure.[7] It can form complexes with acetonitrile and other compounds.[8][9]
References
- ↑ 1.0 1.1 1.2 Perry, Dale L.; Phillips, Sidney L. (1995). Handbook of inorganic compounds. Boca Raton: CRC Press. p. 428. ISBN 0-8493-8671-3. OCLC 32347397.
- ↑ 2.0 2.1 2.2 2.3 Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2017). CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data.. Boca Raton, Florida. p. 104. ISBN 978-1-4987-5429-3. OCLC 957751024.
- ↑ 3.0 3.1 3.2 3.3 Lassner, Erik; Schubert, Wolf-Dieter (1999). Tungsten : Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds. Boston, MA. p. 168. ISBN 1-4615-4907-8. OCLC 1113605323.
- ↑ Panwen Shen, Yunxia Che, Yuji Luo, et al (1998). 无机化学丛书 第八卷.(in Chinese) [lit. Series of Inorganic Chemistry, Vol.8]. Beijing: Science Press. pp 534
- ↑ 5.0 5.1 Mendicino, L.; Electrochemical Society. Dielectric Science and Technology Division; Electrochemical Society. Meeting; Symposium on Environmental Issues with Materials and Processes in the Electronics and Semiconductor Industries (2001). Environmental issues with materials and processes for the electronics and semiconductor industries : proceedings of the fourth international symposium. Pennington, NJ: Electrochemical Society. p. 180. ISBN 1-56677-312-1. OCLC 48710248.
- ↑ Lippard, Stephen J. (1981). Progress in inorganic chemistry. Volume 28. New York, N.Y.: Wiley. p. 245. ISBN 978-0-470-16679-6. OCLC 123541241.
- ↑ Johnson, B. F. G. (1976). Inorganic chemistry of the transition elements. Volume 4, A review of the literature published between October 1973 and September 1974. London: Chemical Society. p. 138. ISBN 978-1-84755-645-5. OCLC 820579758.
- ↑ Levason, William; Reid, Gillian; Zhang, Wenjian (2016). "Coordination complexes of the tungsten(VI) oxide fluorides WOF4 and WO2F2 with neutral oxygen- and nitrogen-donor ligands". Journal of Fluorine Chemistry (Elsevier BV) 184: 50–57. doi:10.1016/j.jfluchem.2016.02.003. ISSN 0022-1139.
- ↑ Arnaudet, Lucile; Bougon, Roland; Charpin, Pierrette; Isabey, Jacques; Lance, Monique; Nierlich, Martine; Vigner, Julien (1989). "Preparation, characterization, and crystal structure of the adducts WOF4.nC5H5N (n = 1, 2)". Inorganic Chemistry (American Chemical Society (ACS)) 28 (2): 257–262. doi:10.1021/ic00301a020. ISSN 0020-1669.

