Chemistry:Tungsten dichloride dioxide
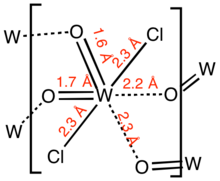
| |
| Names | |
|---|---|
| IUPAC name
Tungsten(VI) dichloride dioxide
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| WO 2Cl 2 | |
| Molar mass | 286.74 g·mol−1 |
| Appearance | Yellow-red crystals |
| Density | 4.67 g/cm3 |
| Melting point | 265 °C (509 °F; 538 K) |
| Boiling point | Sublimes at > 350 °C in vacuum |
| decomposes | |
| Solubility | slightly soluble in ethanol |
| Structure | |
| orthorhombic | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tungsten dichloride dioxide, or Tungstyl chloride is the chemical compound with the formula WO
2Cl
2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO
2Cl
2 is sensitive to moisture, undergoing hydrolysis.
Preparation
WO
2Cl
2 is prepared by ligand redistribution reaction from tungsten trioxide and tungsten hexachloride:
- 2 WO
3 + WCl
6 → 3 WO
2Cl
2
Using a two-zone tube furnace, a vacuum-sealed tube containing these solids is heated to 350 °C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process.[2] An alternative route highlights the oxophilicity of tungsten:[3]
- WCl
6 + 2 ((CH
3)
3Si)
2O → 3 WO
2Cl
2 + 4 (CH
3)
3SiCl
This reaction, like the preceding one, proceeds via the intermediacy of WOCl
4.
Structure
Gaseous tungsten dichloride dioxide is a monomer.[4] Solid tungsten dichloride dioxide is a polymer consisting of distorted octahedral W centres. The polymer is characterized by two short W-O distances, typical for a multiple W-O bond, and two long W-O distances more typical of a single or dative W-O bond.[5]
Related oxy halides
Tungsten forms a number of oxyhalides including WOCl
4, WOCl
3, WOCl
2. The corresponding bromides (WOBr
4, WOBr
3, WOBr
2) are also known as is WO
2I
2.[6]
Reactions
WO
2Cl
2 is a Lewis acid, forming soluble adducts of the type WO
2Cl
2L
2, where L is a donor ligand such as bipyridine and dimethoxyethane. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated in situ from WOCl
4.[7]
References
- ↑ "C&L Inventory". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/132396.
- ↑ Tillack, J. (1973). "Tungsten Oxyhalides". Inorganic Syntheses. 14. 109–122. doi:10.1002/9780470132456.ch22. ISBN 9780470132456.
- ↑ Gibson, V. C.; Kee, T. P.; Shaw, A. (1988). "New, improved synthesis of the group 6 oxyhalides, W(O)Cl4, W(O)2Cl2 and Mo(O)2Cl2". Polyhedron 7 (7): 579–80. doi:10.1016/S0277-5387(00)86336-6.
- ↑ Ward, Brian G.; Stafford, Fred E. (1968). "Synthesis and Structure of Four- and Five-Coordinated Gaseous Oxohalides of Molybdenum(VI) and Tungsten(VI)". Inorganic Chemistry 7 (12): 2569–2573. doi:10.1021/ic50070a020.
- ↑ Jarchow, O.; Schröder, F.; Schulz, H. "Kristallstruktur und Polytypie von WO2Cl2" Zeitschrift für anorganische und allgemeine Chemie 1968, vol. 363, p. 345ff. doi:10.1002/zaac.19683630108
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
- ↑ K. Dreisch, C. Andersson, C. Stalhandske "Synthesis and structure of dimethoxyethane-dichlorodioxo-tungsten(VI)—a highly soluble derivative of tungsten dioxodichloride" Polyhedron 1991, volume 10, p. 2417. doi:10.1016/S0277-5387(00)86203-8
 |