Physics:Capped trigonal prismatic molecular geometry
From HandWiki
| Capped trigonal prismatic molecular geometry | |
|---|---|
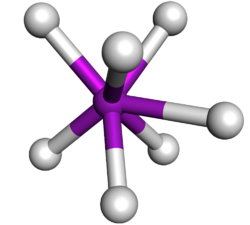 | |
| Examples | TaF2−7 |
| Point group | C2v |
| Coordination number | 7 |
In chemistry, the capped trigonal prismatic molecular geometry describes the shape of compounds where seven atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of an augmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for heptacoordinate transition metal complexes, along with the pentagonal bipyramid and the capped octahedron.[1][2]
Examples of the capped trigonal prismatic molecular geometry are the heptafluorotantalate (TaF2−7) and the heptafluoroniobate (NbF2−7) ions.[3][4]
References
- ↑ Roald. Hoffmann; Barbara F. Beier; Earl L. Muetterties; Angelo R. Rossi (1977). "Seven-coordination. A molecular orbital exploration of structure, stereochemistry, and reaction dynamics". Inorganic Chemistry 16 (3): 511–522. doi:10.1021/ic50169a002.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ Kaupp, Martin (2001). ""Non-VSEPR" Structures and Bonding in d(0) Systems". Angew Chem Int Ed Engl 40 (1): 3534–3565. doi:10.1002/1521-3773(20011001)40:19<3534::AID-ANIE3534>3.0.CO;2-#. PMID 11592184.
- ↑ Zhenyang Lin; Ian Bytheway (1996). "Stereochemistry of Seven-Coordinate Main Group and d0 Transition Metal Molecules". Inorganic Chemistry 35 (3): 594–603. doi:10.1021/ic950271o.
 |
