Physics:Pentagonal pyramidal molecular geometry
From HandWiki
Short description: Molecular geometry
| Pentagonal pyramidal molecular geometry | |
|---|---|
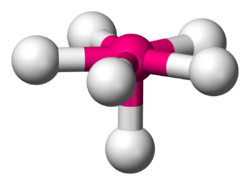 | |
| Examples | XeOF−5 |
| Point group | C5v |
| Coordination number | 6 |
| Bond angle(s) | 90°, 72° |
| μ (Polarity) | >0 |
In chemistry, pentagonal pyramidal molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are arranged around a central atom, at the vertices of a pentagonal pyramid. It is one of the few molecular geometries with uneven bond angles.[1]
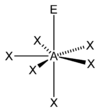
Examples
References
- ↑ Baran, Enrique J. (2008). "Mean amplitudes of vibration of molecules and ions with interhalogen bonds and related species". Journal of Fluorine Chemistry 129 (11): 1060–1072. doi:10.1016/j.jfluchem.2008.06.016. Bibcode: 2008JFluC.129.1060B.
- ↑ 2.0 2.1 Baran, E. (2000). "Mean amplitudes of vibration of the pentagonal pyramidal XeOF−5 and IOF2−5 anions". J. Fluorine Chem. 101: 61–63. doi:10.1016/S0022-1139(99)00194-3.
- ↑ Housecroft, Catherine E.; Sharpe, Alan G. (2005). Inorganic Chemistry (2nd ed.). Pearson Prentice-Hall. p. 485. ISBN 0130-39913-2.
Pentagonal pyramid, Wolfram MathWorld
 |
