Physics:Pentagonal planar molecular geometry
From HandWiki
| Pentagonal planar molecular geometry | |
|---|---|
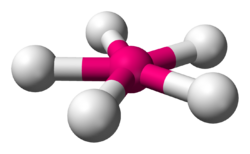 | |
| Examples | XeF5− |
| Point group | D5h |
| Coordination number | 5 |
| Bond angle(s) | 72° |
| μ (Polarity) | 0 |
In chemistry, the pentagonal planar molecular geometry describes the shape of compounds where five atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a pentagon.

Examples
The only two pentagonal planar species known are the isoelectronic (nine valence electrons) ions XeF−5 and IF2−5.[1] Both are derived from the pentagonal bipyramid with two lone pairs occupying the apical positions and the five fluorine atoms all equatorial.
References
- ↑ Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 498. ISBN 978-0130399137.
 |
