Physics:Cataract surgery
| Cataract surgery | |
|---|---|
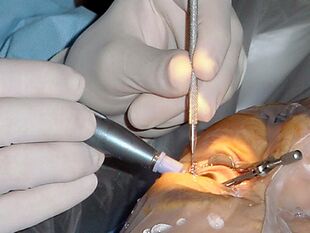 Cataract surgery, using a temporal approach phacoemulsification probe (in right hand) and "chopper" (in left hand) | |
| Specialty | Ophthalmology |
| ICD-9-CM | 13.19 |
| MeSH | D002387 |
| MedlinePlus | 002957 |
Cataract surgery, which is also called lens replacement surgery, is the removal of the natural lens (also called the "crystalline lens") of the human eye that has developed a cataract, an opaque or cloudy area.[1] The eye's natural lens is usually replaced with an artificial intraocular lens (IOL).[2]
Over time, metabolic changes of the crystalline lens fibres lead to the development of a cataract, causing impairment or loss of vision. Some infants are born with congenital cataracts, and environmental factors may lead to cataract formation. Early symptoms may include strong glare from lights and small light sources at night, and reduced visual acuity at low light levels.[3][4]
During cataract surgery, the cloudy natural lens is removed, either by emulsification in place or by cutting it out.[2] An IOL is usually implanted in its place to restore useful focus. Cataract surgery is generally performed by an ophthalmologist in an out-patient setting at a surgical centre or hospital. Local anaesthesia is normally used; the procedure is usually quick, and causes little or no pain and minor discomfort to the patient. Recovery sufficient for most daily activities usually takes place in days and full recovery about a month.[5]
Well over 90% of operations are successful in restoring useful vision and there is a low complication rate. Day care, high volume, minimally invasive, small incision phacoemulsification with quick post-operative recovery has become the standard of care in cataract surgery in the developed world.[2] Manual small incision cataract surgery (MSICS), which is considerably more economical in time, capital equipment and consumables but provides comparable results is popular in the developing world.[6] Both procedures have a low risk of serious complications.[7][8]
Description
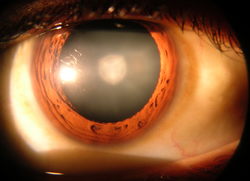
Cataract surgery, also called lens replacement surgery, is the removal of the natural lens (also called the "crystalline lens") of the human eye that has developed a cataract, an opaque or cloudy area.[1] The eye's natural lens is usually replaced with an artificial intraocular lens (IOL) to restore focus within a useful range of distances.[2] It is the definitive treatment for vision impairment due to lens opacification.
A cataract is a cloudy area in the lens of the eye that causes visual impairment.[4][9] Cataracts usually develop slowly and can affect one or both eyes.[4] Symptoms may include faded colours, blurry or double vision, halos around lights, sensitivity to glare from bright lights, and night blindness.[4] This may result in difficulty driving, reading, or recognizing faces.[10] Poor vision caused by cataracts may also increase the risk of falling and depression.[11]
Cataracts are most commonly due to aging but may also be caused by trauma or radiation exposure, be present from birth, or may occur following eye surgery for other problems.[4][12] Risk factors include diabetes, longstanding use of corticosteroid medication, smoking tobacco, and extended hyperbaric oxygen therapy, prolonged exposure to sunlight, and consumption of alcohol.[4] Cataracts form when clumps of protein or yellow-brown pigment accumulate in the lens, which reduces transmission of light to the retina at the back of the eye.[4] Cataracts are diagnosed by an eye examination.[4]
Cataract surgery is needed only if the cataracts are causing the person problems. Early symptoms may be improved with glasses;[4] if this does not help, cataract surgery is the only effective treatment.[4] Surgery with implants generally results in better vision and an improved quality of life. The procedure is not readily available in many countries.[4][13][12][14]
About 20 million people worldwide are blind due to cataracts,[12] which cause 51% of all cases of blindness and 33% of visual impairment.[15][16] They are the cause of approximately 5% of blindness in the United States, and nearly 60% of blindness in parts of Africa and South America.[14] Blindness from cataracts occurs in between 10 and 40 children per 100,000 in the developing world, and between 1 and 4 children per 100,000 in the developed world.[9] Cataracts become more common with age.[4] In the United States, cataracts occur in 68% of those over the age of 80 years.[17] They are more common in women, and less common in Hispanic and Black people.[17]
Contraindications
Contraindications to cataract surgery include cataracts that do not cause visual impairment and medical conditions that predict a high risk of unsatisfactory surgical outcomes.[2] Implantation of a posterior chamber intraocular lens (PCIOL) in patients younger than seven months is controversial due to rapid growth of the eyes at this age. Eye growth in infants is unpredictable; an early implant is likely to result in large refractive error later in childhood.[18] Congenital cataracts may be caused by factors that make the eye more susceptible to an excessive amount of inflammation, which may be difficult to control.[19] Intraocular lenses (IOLs) are associated with a greater risk of visual axis opacities in this age group; aphakic optical correction in patients without using an IOL is usually managed with either contact lenses or glasses. Implantation of an IOL in a second operation may be considered later.[18]
Technique
Two main types of surgical procedures are in common use throughout the world. In phacoemulsification (phaco), the natural lens is broken into small pieces and removed by suction, and in extracapsular cataract extraction (ECCE), the lens is removed from its capsule and extracted from the eye whole or a small number of substantial pieces. In most surgeries, an IOL is inserted. Foldable lenses are generally used for the 2–3 mm (0.08–0.12 in) phaco incision, while non-foldable lenses can be placed through the larger extracapsular incision. The small incision size used in phacoemulsification generally allows for sutureless incision closure. ECCE uses a larger incision of 10–12 mm (0.39–0.47 in) and usually requires stitches; this requirement led to a variation of ECCE known as manual small incision cataract surgery (MSICS), which does not usually need stitches.[2]
Cataract surgery using intracapsular cataract extraction (ICCE) has been superseded by phacoemulsification and MSICS, and is rarely performed.[2] ECCE has largely become a contingency procedure to deal with complications during surgery.[20] Phacoemulsification is the most-commonly performed cataract procedure in the developed world. The high capital and maintenance costs of a phacoemulsification machine and of the associated disposable equipment, however, has meant ECCE and MSICS remain the most-commonly performed procedures in developing countries.[2] Cataract surgery is commonly done as an outpatient or day care procedure, which is cheaper than hospitalisation and an overnight stay, and there is evidence day surgery has similar medical outcomes.[21]
Types of surgery
The main types of surgical technique used in cataract surgery are phacoemulsification and manual extraction.
- Phacoemulsification involves the use of a machine with an ultrasonic handpiece equipped with a titanium or surgical steel tip which vibrates at an ultrasonic frequency—commonly 40 kHz—to emulsify the lens tissue. A second instrument, which is sometimes called a "cracker" or "chopper", may be used from a side port to break the hard cataract nucleus into smaller pieces, making emulsification and the aspiration of cortical material—the soft part of the lens around the nucleus—easier. After phacoemulsification of the lens nucleus and cortical material is completed, a dual irrigation-aspiration (I-A) probe or a bimanual I-A system is used to remove the remaining peripheral cortical material.
- Femtosecond laser-assisted cataract surgery has been reported to be safe, and may have less adverse effect on the cornea and macula than manual phacoemulsification. The laser is used to make the corneal incision, the capsulotomy and to initiate lens fragmentation, which reduces the energy requirements for phacoemulsification. It offers high precision, effective lens fragmentation at lower power levels and good optical quality, but as of 2022 has not been shown to have significant visual, refractive or safety benefit over manual phacoemulsification, and it has a higher cost.[2][22][23]
- Extracapsular cataract extraction (ECCE), also known as manual extracapsular cataract extraction, is the removal of almost the entire natural lens in one piece, while the elastic lens capsule (posterior capsule) is left intact to allow implantation of an intraocular lens.[2] The lens is manually removed through a 10–12 mm (0.39–0.47 in) incision in the cornea or sclera. Although it requires a larger incision and the use of stitches, this method may be preferable for patients with very hard cataracts or other situations in which phacoemulsification is problematic.[20]
- Manual small incision cataract surgery (MSICS) is an evolution of ECCE; the entire lens is removed from the eye through a self-sealing scleral tunnel wound. A well-constructed scleral tunnel is held closed by internal pressure, is watertight, and does not require suturing. The wound is relatively smaller than that in ECCE but is still markedly larger than a phaco wound. Comparative trials of MSICS against phaco in dense cataracts have found no difference in outcomes but MSICS had shorter operating times and significantly lower costs.[6] MSICS has become the method of choice in the developing world because it provides high-quality outcomes with less surgically induced astigmatism than ECCE, no suture-related problems, quick rehabilitation, and fewer post-operative visits. MSICS is easy and fast to learn for the surgeon, cost effective, simple, and applicable to almost all types of cataract.[8]

- Intracapsular cataract extraction (ICCE) is the removal of the lens and the surrounding lens capsule in one piece. The procedure has a relatively high rate of complications due to the large incision required and pressure placed on the vitreous body. It has therefore been largely superseded, and is rarely performed in countries where operating microscopes and high-technology equipment are readily available.[2] After lens removal by ICCE, an intraocular lens implant can be placed in either the anterior chamber or sutured into the ciliary sulcus.[7] Cryoextraction is a technique used in ICCE; the cataract is extracted using a cryoprobe, the refrigerated tip of which adheres to the tissue of the lens at the contact point by freezing with a cryogenic substance such as liquid nitrogen, facilitating its removal.[24] Cryoextraction may still be used for the removal of subluxated lenses.[25]
- Refractive lens exchange is effectively the same procedure used to replace a lens with high refractive error when other methods are not effective. There are risks in addition to cataract procedural risks.[2] A related procedure is the implantation of phakic intraocular lenses in series with the natural lens to correct vision in cases of high refractive errors.[26]
Ophthalmic viscosurgical devices
Ophthalmic viscosurgical devices (OVDs) are a class of clear, gel-like materials used in cataract surgery to maintain the volume and shape of the anterior chamber of the eye, and protect the intraocular tissues during the procedure. They were originally called viscoelastic substances or viscoelastics. Their consistency allows surgical instruments to move through them but when there is low shear stress, they do not flow and retain their shape. OVDs are available in several formulations, which may be combined or used individually as best suits the procedure. OVDs are introduced into the anterior chamber at the start of the procedure and removed at the end, when they are replaced by a buffered saline solution.[7] The tendency of cohesive OVDs to adhere to themselves helps with removal.[27]
Intraocular lenses


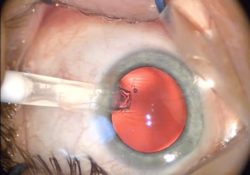
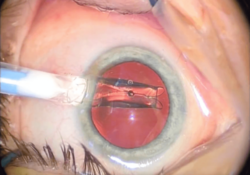
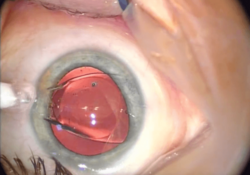
After the removal of a cataract, an intraocular lens (IOL) is usually implanted into the eye to provide refractive compensation for the lack of the natural lens. A foldable IOL may be implanted through a 1.8 to 2.8 mm (0.071 to 0.110 in) incision, whereas a rigid poly(methyl methacrylate) (PMMA) lens requires a larger cut. Foldable IOLs are made of silicone, hydrophobic, or hydrophilic acrylic material of appropriate refractive power; it is folded using a holder/folder or a proprietary insertion device provided with the IOL.[28] The IOL is inserted through the incision, usually into the capsular bag within the posterior chamber (in-the-bag implantation). Sometimes, a sulcus implantation—in front or on top of the capsular bag but behind the iris—may be required because of posterior capsular tears or zonulodialysis. This requires a modification of refractive power because of the more-anterior placement on the optical axis.[29]
Other designs of multifocal intraocular lens that focus light from distant and near objects, working with similar effect to bifocal or trifocal eyeglasses, are available. Preoperative patient selection and good counselling is necessary to avoid unrealistic expectations and post-operative patient dissatisfaction, and possibly a requirement to replace the lens.[30] Acceptability of these lenses has improved and studies have shown good results in patients selected for expected compatibility.[31]
One model of accommodating lens has two hinged struts on opposite edges that displace the lens along the optical axis when an inward transverse force is applied to the haptic loops at the outer ends of the struts—the components transferring the movement of the contact points to the device—and it recoils when the force is reduced. It is implanted in the eye's lens capsule, where the contractions of the ciliary body, which would focus the eye with the natural lens, are used to focus the implant.[32][2]
IOLs used in correcting astigmatism have different curvature on two orthogonal axes, as on the surface of a torus, and are called toric lenses. The STAAR Surgical Intraocular Lens was the first-such lens developed in the United States; it may correct up to 3.5 dioptres. Alcon created a different model of toric lens that may correct up to 3 dioptres of astigmatism. To achieve the most benefit from a toric lens, the surgeon must place the lens with the axes of curvature to suit the patient's astigmatism. Intraoperative aberrometry[Note 1] can be used to assist the surgeon in toric lens placement and minimize astigmatic errors.[33][34]
Monofocal IOLs provide accurately focused vision at one distance only; far, intermediate, or near. Patients who are fitted with these lenses may need to wear glasses or contact lenses while reading or using a computer. These lenses usually have uniform spherical curvature.[35][36]
Cataract surgery may be performed to correct vision problems in both eyes; if both eyes are suitable, patients are usually advised to consider monovision. This procedure involves inserting into one eye an IOL that provides near vision and one that provides distance vision in the other. Although most patients can adjust to having monofocal IOLs with differing focal length, some cannot compensate and may experience blurred vision at both near and far distances. An IOL optimised for distance vision may be combined with an IOL that optimises intermediate vision instead of near vision as a variation of monovision.[28]
The first aspheric IOLs were developed in 2004; these have a flatter periphery than the middle of the lens, improving contrast sensitivity. The effectiveness of aspheric IOLs depends on a range of conditions and they may not always provide significant benefit.[37]
Some IOLs absorb ultraviolet and high-energy blue light. The natural crystalline lens of the eye filters these potentially harmful frequencies. A Cochrane review of 2018 found there is unlikely to be a significant difference in distance vision between blue-filtering and plain lenses, and was unable to identify a difference in contrast sensitivity or colour discrimination.[38][39]
The light-adjustable IOL was approved by the U.S. Food and Drug Administration (FDA) in 2017.[40] This type of IOL is implanted in the eye and then treated with ultraviolet light to alter the curvature of the lens.[41]
In some cases, it may be necessary or desirable to insert an additional lens over the already implanted one, also in the posterior capsule. This type of IOL placement is called "piggyback" IOLs and is usually considered when the visual outcome of the first implant is not optimal.[42] In such cases, implanting another IOL over the existing one is considered safer than replacing the initial lens. This approach may also be used in patients who need high degrees of vision correction.[43]
The appropriate refractive power of the IOL must be selected, much like a spectacle lens prescription, to provide the desired refractive outcome. Preoperative measurements, including corneal curvature, axial length, and white-to-white measurements are used to estimate the required power of the IOL. These methods include several formulae, including Hagis,[44] Hoffer Q,[44] Holladay 1,[44] Holladay 2,[44] and SRK/T.[45] Free online calculators use similar input data.[44] A history of LASIK surgery requires different calculations to take this into account.[44] Refractive results using power calculation formulae based on pre-operative biometrics leave patients within 0.5 dioptres of target (correlates to visual acuity of 6/7.5 (20/25) when targeted for distance) in 55% of cases and within one dioptre (correlates to 6/12 (20/40) when targeted for distance) in 85% of cases. Developments in intraoperative wavefront technology have demonstrated power calculations that provide improved outcomes, yielding 80% of patients within 0.5 dioptres (7/7.5 (20/25) or better).[34]
Cost is an important aspect of these lenses. Although Medicare covers the cost of monofocal IOLs in the United States, patients will have to pay the price difference if they choose more expensive lenses.[46]
Preoperative evaluation
An eye examination or pre-operative evaluation by an eye surgeon is necessary to confirm the presence of a cataract and to determine the patient's suitability for surgery. The patient must fulfil certain requirements:
- The degree of reduction of vision due largely to the cataract should be evaluated. While the existence of other sight-threatening diseases, such as age-related macular degeneration or glaucoma, does not preclude cataract surgery, less improvement may be expected in their presence.[2]
- The eyes should have a normal pressure, or any glaucoma should be adequately controlled with medications. In cases of uncontrolled glaucoma, a combined cataract-glaucoma procedure (phaco-trabeculectomy) can be planned and performed.[47]
- The pupil should be adequately dilated using eyedrops; if pharmacologic pupil dilation is insufficient, procedures for mechanical pupil dilatation may be needed during the surgery.[48]
- Patients with retinal detachment may be scheduled for a combined vitreo-retinal procedure, along with PCIOL implantation.[49]
- Patients taking tamsulosin (Flomax), a common drug for enlarged prostate, are prone to developing a surgical complication known as intraoperative floppy iris syndrome (IFIS), which must be correctly managed to avoid the complication posterior capsule rupture;[Note 2] prospective studies have shown the risk is greatly reduced if the surgeon is informed of the patient's history with the drug, and has appropriate alternative techniques prepared.[50]
- A Cochrane Review of three randomized clinical trials, including over 21,500 cataract surgeries, examined whether routine preoperative medical testing resulted in a reduction of adverse events during surgery. Results showed performing preoperative medical testing did not result in a reduction of risk of intraoperative or postoperative medical adverse events, compared to surgeries with no or limited preoperative testing.[51]
Operation procedures
Antibiotics may be administered pre-operatively, intra-operatively, or post-operatively. Frequently, a topical corticosteroid or nonsteroidal anti-inflammatory drug (NSAID) is used in combination with topical antibiotics post-operatively.[7]
Most cataract operations are performed under local anaesthetic, allowing the patient to go home the same day. The use of an eye patch may be indicated, usually for some hours and while sleeping, after which the patient is instructed to use anti-inflammatory eyedrops to control inflammation, and antibiotic eyedrops to prevent infection. Lens and cataract procedures are commonly performed in an outpatient setting; in the United States, 99.9% of lens and cataract procedures in 2012 were done in an outpatient setting.[52][7]
Occasionally, a peripheral iridectomy may be performed to minimize the risk of pupillary block glaucoma.[7] Surgical iridectomy, an opening through the iris can be fashioned manually or with a neodymium-doped yttrium aluminium garnet (Nd:YAG) laser. Laser peripheral iridotomy may be performed either before or following cataract surgery.[53]
The iridectomy hole is larger when done manually than when performed with a laser. When the manual surgical procedure is performed, some negative side-effects, such as the opening of the iris being visible to others, and the passage of light into the eye through the new hole, creating visual disturbance. In the case of visual disturbance, the eye and brain often learn to compensate and ignore the effects over two months. Sometimes, the peripheral iris opening can heal and the hole ceases to exist.[53][54]
The most-commonly used procedures are phacoemulsification and manual small incision cataract surgery (MSICS). In either of these procedures, it can sometimes be necessary to convert to ECCS to deal with a problem better managed through a larger incision.[20]
Phacoemulsification
The surgical procedure in phacoemulsification for removal of cataract is typically performed under an operating microscope. Either topical, sub-tenon, peribulbar, or retrobulbar local anaesthesia is used, usually causing the patient little or no discomfort.[55] Topical anaesthetics are most commonly used; these are placed on the globe of the eye as eyedrops before surgery or in the globe during surgery.[56] Local-anaesthetic injection techniques include sub-conjunctival injections and injections behind the globe (retrobulbar block) to block regional nerves and prevent eye movement.[7] Intravenous sedation may be combined with the local anaesthetic. General anaesthesia and retrobulbar blocks were historically used for intracapsular cataract surgery, but local and topical anaesthesia are commonly used for small incision surgery and phacoemulsification.[2]
The operation site is prepared using disinfection; this includes the area around the eye and the rest of the face, and exposure of the eyeball using an eyelid speculum;[57]
Entry into the eye is made through a minimal incision;[7] the incision for cataract surgery has evolved along with the techniques for cataract removal and IOL placement. In phacoemulsification, the size depends on the requirements for IOL insertion. A more-posterior incision simplifies wound closure and decreases induced astigmatism but is more likely to damage blood vessels. With foldable IOLs, it is sometimes possible to use incisions smaller than 3.5 mm (0.14 in). The shape, position, and size of the incision affect the capacity for self sealing, the tendency to induce astigmatism, and the surgeon's ability to manoeuvre instruments through the opening.[7] One or two smaller side-port incisions at 60-to-90 degrees from the main incision may be needed to access the anterior chamber with additional instruments.[57]
Ophthalmic viscosurgical devices (OVDs), also known as viscoelastics, are injected into the anterior chamber to support, stabilize, and protect the eyeball to help maintain eye shape and volume, and to distend the lens capsule during IOL implantation.[27] In capsulorhexis, a circular opening is made on the front surface of the lens capsule to access the lens within. In phacoemulsification, an anterior continuous curvilinear capsulorhexis is used to create a round, smooth-edged opening through which the lens nucleus can be emulsified and the intraocular lens implant inserted.[58]
The cataract's outer (cortical) layer is then separated from the capsule by a gentle, continuous flow or pulsed dose of liquid from a cannula, which is injected under the anterior capsular flap along the edge of the capsulorhexis opening. This step is called hydrodissection.[59][60] In hydrodelineation, fluid is injected into the body of the lens through the cortex against the nucleus of the cataract, which separates the hardened nucleus from the softer cortex shell by flowing along the interface between them. The smaller hard nucleus can then be more-easily phacoemulsified. The posterior cortex serves as a buffer, protecting the posterior capsule membrane. The smaller size of the separated nucleus requires shallower and less-peripheral grooving, and produces smaller fragments after cracking or chopping. The posterior cortex also maintains the shape of the capsule, which reduces the risk of posterior capsule rupture.[61]
After nuclear cracking or chopping (if needed), the cataract is destroyed using ultrasound and the remaining lens cortex (outer layer of lens) material from the capsular bag is carefully aspired and in capsular polishing, the remaining epithelial cells from the capsule are removed if necessary.[62] The folded intraocular replacement lens is implanted, usually into the remaining posterior capsule, and correct unfolding is ensured. Aligning the IOL in the correct axis to counteract astigmatism is necessary for a toric IOL.[2]
OVDs that were injected to stabilize the anterior chamber, protect the cornea from damage, and distend the cataract's capsule during IOL implantation are removed from the eye to prevent post-operative viscoelastic glaucoma, a severe intra-ocular pressure increase. This is done via suction from the irrigation-aspiration instrument and replacement by buffered saline solution (BSS). Removal of OVDs from behind the implant reduces the risk and magnitude of postoperative pressure spikes or capsular distention.[7]
In the final step, the wound is sealed by elevating the pressure inside the globe with BSS, which presses the internal tissue against the external tissue of the incision, holding the incision closed. If this does not achieve a satisfactory seal, a suture may be added. The wound is then hydrated.[7]
Manual small incision cataract surgery (MSICS)
Many of the steps are similar and some may be the same as for phacoemulsification; the main differences are related to the alternative method of incision and cataract extraction from the capsule and eye. Preparation may begin three-to-seven days before surgery with the preoperative application of NSAIDs and antibiotic eye drops.[8] The pupil is dilated using drops if the IOL is to be placed behind the iris to help better visualise the cataract. Pupil-constricting drops are reserved for secondary implantation of the IOL in front of the iris, when the cataract has already been removed without primary IOL implantation. Anaesthesia may be placed topically as eyedrops or injected next to (peribulbar) or behind (retrobulbar) the eye[56] or sub-tenons. Local anaesthetic nerve blocking has been recommended to facilitate surgery.[8] Topical anaesthetics may be used at the same time as a intracameral lidocaine injection to reduce pain during the operation.[56] Oral or intravenous sedation may also be used to reduce anxiety. General anaesthesia is rarely necessary but may be used for children and adults with medical or psychiatric issues affecting their ability to remain still during the procedure.[56][7]
The operation may occur on a stretcher or a reclining examination chair. The eyelids and surrounding skin are swabbed with a disinfectant, such as 10% povidone-iodine, and topical povidone-iodine is put in the eye. The face is covered with a cloth or sheet with an opening for the operative eye. The eyelid is held open with a speculum to minimize blinking during surgery.[57] Pain is usually minimal in properly anaesthetised eyes, though a pressure sensation and discomfort from the bright operating microscope light is common.[7] Bridle sutures[Note 3] may be used to help to stabilize the eyeball during sclerocorneal tunnel incision, and during extraction of the nucleus and epinucleus through the tunnel.[8]
The small incision into the anterior chamber of the eye is made at or near the corneal limbus, where the cornea and sclera meet, either superior or temporal.[8] Advantages of the smaller incision include use of few-or-no stitches and shortened recovery time.[2] The "small" incision is small in comparison with the earlier ECCE incision but considerably larger than the phaco incision. The precise geometry of the incision is important as it affects the self-sealing of the wound and can cause astigmatism by distortion of the cornea during healing. A sclerocorneal or scleral tunnel incision is commonly used, which reduces induced astigmatism if suitably formed.[6][57] A sclerocorneal tunnel, a three-phase incision, starts with a shallow incision perpendicular to the sclera, followed by an incision through the sclera and cornea approximately parallel to the outer surface, and then a beveled incision into the anterior chamber. This structure provides the self-sealing characteristic because internal pressure presses together the faces of the incision.[8]
The depth of the anterior chamber and position of the posterior capsule may be maintained during surgery by OVDs or an anterior chamber maintainer, which is an auxiliary cannula providing a sufficient flow of BSS to maintain the stability of the shape of the chamber and internal pressure.[63][64] Using an instrument called a cystotome, capsulotomy, also known as a cystotomy, is made to open the surface of the lens capsule .[65] The continuous curvilinear capsulorhexis technique is in common use. An anterior capsulotomy is the opening of the front portion of the lens capsule, providing access to remove the cataract,[66] whereas a posterior capsulotomy is an opening of the back portion of the lens capsule, which is not usually necessary or desirable unless it has opacified.[67] The types of capsular openings commonly used in MSICS are the continuous curvilinear capsulorhexis, the can-opener capsulotomy, and the envelope capsulotomy.[63]
The cataract lens is then removed from the capsule and anterior chamber using hydroexpression[Note 4] viscoexpression,[Note 5] or more-direct mechanical methods.[63][68][69] Following cataract removal, an IOL is usually inserted into the posterior capsule.[7] When the posterior capsule is damaged, the IOL may be inserted into the ciliary sulcus,[29] or a glued intraocular lens technique may be applied.[70] After the IOL is inserted, the ophthalmic viscosurgery device is aspirated and replaced with BSS, and the wound is closed. The surgeon checks the incision does not leak fluid because wound leakage increases the risk of microorganisms gaining access into the eye and predisposing it to endophthalmitis. An antibiotic/steroid combination eye drop is put in, and an eye shield may be applied, sometimes supplemented with an eye patch.[7]
Converting to ECCE to manage a contingency
Sometimes a change from a phacoemulsification procedure to ECCE may be necessary.[20] This may occur in the event of posterior capsule rupture, zonular dehiscence,[Note 6] or a dropped nucleus[Note 7] with a nuclear fragment more than half the size of the cataract;[20] problematic capsulorhexis with a hard cataract;[20] or a very dense cataract where phacoemulsification is likely to cause permanent damage to the cornea.[20] Similarly, a change from MSICS to ECCE is appropriate when[20] the nucleus is too large for the MSICS incision;[20] and in cases where the nucleus is found to be deformed during MSICS on a nanophthalmic (very small) eye.[Note 8][20]
Complications
Complications can develop during and after surgery.
During surgery
Posterior capsular rupture, a tear in the posterior capsule of the natural lens, is the most-common complication during cataract surgery, with a rate of around 0.5% to 5.2%.[2] Surgical management may involve anterior vitrectomy and occasionally, alternative planning for implanting the IOL, either in the ciliary sulcus—the space between the iris and the ciliary body—in the anterior chamber in front of the iris, or less commonly, sutured to the sclera. Posterior capsule rupture can cause lens fragments to be retained, corneal oedema, and cystoid macular oedema; it is also associated with a six-times increase of risk of endophthalmitis and as much as 19 times increase in the risk of retinal detachment.[2][71] Management methods include the Intraocular lens scaffold procedure.[72]
Suprachoroidal hemorrhage is a rare complication of intraocular surgery where the ciliary arteries bleed into the space between the choroid and the sclera.[73] It is a potentially vision-threatening pathology. Risk factors for suprachoroidal hemorrhage include anterior chamber intraocular lens (ACIOL), axial myopia, advanced age, atherosclerosis, glaucoma, systolic hypertension, tachycardia, uveitis, and previous ocular surgery. Immediate and effective management of a suprachoroidal hemorrhage is necessary to protect vision.[7]
Intraoperative floppy iris syndrome has an incidence of around 0.5% to 2.0%.[2] Iris or ciliary body injury has an incidence of about 0.6%-1.2%.[2] In the event of a posterior capsule rupture, fragments of the nucleus can find their way through the tear into the vitreous chamber; this is called posterior dislocation of nuclear fragments. Recovery of the fragments is not always desirable and it is rarely successful. The rest of the fragments should generally be stabilised first, and vitreous prevented from entering the anterior chamber. Removal of the fragments may be best referred to a vitreoretinal specialist.[7]
Other complications include failure to aspirate all lens fragments, leaving some in the anterior chamber;[71] and incisional burns caused by overheating of the phacoemulsification tip when ultrasonic power continues while the irrigation or aspiration lines are blocked—the flow through these lines is used to keep the tip cool. Burns to the incision may make closure difficult and can cause corneal astigmatism.[7]
After surgery
Complications after cataract surgery are relatively uncommon. Posterior vitreous detachment (PVD) does not directly threaten vision but is of increasing interest because the interaction between the vitreous body and the retina might play a decisive role in the development of major pathological vitreoretinal conditions. PVD may be more problematic with younger patients because many patients older than 60 have already gone through PVD. PVD may be accompanied by peripheral light flashes and increasing numbers of floaters.[74]
Some people develop a posterior capsular opacification (PCO), also called an after-cataract. As a physiological change expected after cataract surgery, the posterior capsular cells undergo hyperplasia and cellular migration, showing up as a thickening, opacification and clouding of the posterior lens capsule, which is left behind after the cataract is removed, for placement of the IOL. This may compromise visual acuity, and can be safely and painlessly corrected using a laser to make a hole in the posterior lens capsule. It usually is a quick outpatient procedure that uses a Nd-YAG laser to clear the central portion of the opacified posterior pole of the capsule (posterior capsulotomy).[75] This creates a clear central visual axis to improve visual acuity.[76] In very thick opacified posterior capsules, a manual surgical capsulectomy may be needed. A posterior capsulotomy must be taken in consideration in the event of IOL replacement because vitreous can migrate toward the anterior chamber through the opening previously occluded by the IOL. Posterior capsule opacification has an incidence of about 0.3% to 28.4%.[2]
Retinal detachment normally occurs at a prevalence of 1 in 1,000 (0.1%) but patients who have had cataract surgery are at an increased risk (0.5–0.6%) of developing rhegmatogenous retinal detachment (RRD)—the most-common form of retinal detachment.[77] Cataract surgery increases the rate of vitreous humour liquefaction, which leads to increased rates of RRD.[78] When a retinal tear occurs, vitreous liquid enters the space between the retina and retinal pigmented epithelium (RPE), and presents as flashes of light (photopsia), dark floaters, and loss of peripheral vision.[77] Toxic anterior segment syndrome (TASS), a non-infectious inflammatory condition, may occur following cataract surgery. It is usually treated with topical corticosteroids in high dosage and frequency.[79]
Endophthalmitis is a serious infection of the intraocular tissues, usually following intraocular surgery complications or penetrating trauma, and one of the most-severe. It is rare in cataract surgery due to the use of prophylactic antibiotics, There is some concern the clear cornea incision might predispose to the increase of endophthalmitis but no conclusive study has corroborated this suspicion.[80] An intracameral injection of antibiotics may be used as a preventive measure. A meta-analysis showed the incidence of endophthalmitis after phacoemulsification to be 0.092%. The risk is higher with diabetes, advanced age, larger incision procedures,[28] and vitreous communication with the anterior chamber due to posterior capsule rupture. The risk of vitreous infection is at least six times higher than for the aqueous.[81] Typical presentation is within two weeks after the procedure with decreased visual acuity, red-eye, and pain. Hypopyon occurs about 80% of the time. Common infective agents include coagulase-negative staphylococci and Staphylococcus aureus in about 80% of infections. Management includes vitreous humour tap and injection of broad-spectrum antibiotics. Outcomes can be severe even with treatment, and may range from permanently decreased visual acuity to no light perception, depending on the microbiological etiology.[2]
Glaucoma may occur and may be very difficult to control. It is usually associated with inflammation, especially when small fragments or chunks of the nucleus access the vitreous cavity. Some experts recommend early intervention by posterior pars plana vitrectomy when this condition occurs. In most cases, raised post-operative intraocular pressure is transient and benign, usually returning to baseline within 24 hours without intervention. Glaucoma patients may experience further visual field loss or a loss of fixation, and are more likely to experience intraocular pressure spikes.[82] Secondary glaucoma is an important complication of surgery for congenital cataracts. It can occur several years after cataract surgery so these patients need lifelong surveillance.[83]
Mechanical pupillary block is shallowing of the anterior chamber by obstruction of the flow of aqueous humour flow through the pupil by the vitreous face or IOL;[84] This can occur when contact between the edge of the pupil and an adjacent structure blocks the flow of aqueous through the pupil. The iris then bulges forward and closes the angle between the iris and cornea, blocking drainage through the trabecular meshwork and causing an increase in intraocular pressure. This is more frequent as a complication of anterior chamber intraocular lens implantation, but has been known to occasionally occur with posterior IOL implantation.[85]
Swelling of the macula, the central part of the retina, results in macular oedema and can occur a few days or weeks after surgery. Most such cases can be successfully treated. Preventative use of nonsteroidal anti-inflammatory drugs has been reported to reduce the risk of macular oedema to some extent.[86] Uveitis–glaucoma–hyphema syndrome is a complication caused by the mechanical irritation of a mis-positioned IOL over the iris, ciliary body or iridocorneal angle.[87]
Other possible complications include Elevated intraocular pressure;[84] swelling or oedema of the cornea, which is sometimes associated with transient or permanent cloudy vision (pseudophakic bullous keratopathy); displacement or dislocation of the IOL implant (rare); unplanned high refractive error—either myopic or hypermetropic—due to error in the ultrasonic biometry (measurement of the eye length and calculation of the required intraocular lens power); cyanopsia, in which the patient's vision tinted blue and often occurs for a few days, weeks or months after removal of a cataract; and floaters, which commonly appear after surgery.[39]
It may be necessary to exchange,[Note 9] remove[Note 10] or reposition[Note 11] an IOL after surgery, for any of several reasons:[84]
- Capsular block syndrome, which is hyper-distention of the lens capsular bag due to the IOL blocking fluid from draining through the anterior capsulotomy. This may cause a myopic refractive error;[84]
- Chronic anterior uveitis, which is a persistent inflammation of the anterior segment;[84]
- Chronic loss of endothelial cells faster than the rate due to normal aging;[84]
- Iris pigment epithelium loss;[84]
- Pain;[84]
- Progressive elongation of the pupil in direction of the long axis of the IOL;[84]
- Progressive closing of the anterior chamber angle due to propagation of anterior synechiae without apparent anterior uveitis;[84]
- Incorrect IOL refractive power;[84]
- Incorrect positioning of the IOL,including decentring, tilt, or rotation that reduces correct function;[84]
- Damage or deformation of the IOL;[84]
- Unexpected optical results due to defects of the IOL;[84]
- Undesirable optical phenomena reported by the patient due to any other cause.[84]
Risk
Statistically, cataract surgery and IOL implantation are procedures with the safest and highest success rates in eye care.[7] As with any type of surgery, some level of risk remains. As of 2011, cataract surgery is the most-frequently performed surgical procedure in the United States, with 1.8 million Medicare beneficiaries undergoing the procedure in 2004. This rate is expected to increase as the population ages.[88]
Most complications of cataract surgery do not result in long-term visual impairment but some severe complications can result in irreversible blindness.[88] A survey of adverse results affecting Medicare patients recorded between 2004 and 2006 showed an average rate of 0.5% for one or more severe post-operative complications, with the rate decreasing by about 20% over the study period. The most important risk factors identified were diabetic retinopathy and a combination of cataract surgery with another intraocular procedure on the same day. In the study, 97% of the surgeries were not combined with other intraocular procedures; while 3% were combined with retinal, corneal or glaucoma surgery on the same day.[88]
Recovery and rehabilitation

Following cataract surgery, side-effects such as grittiness, watering, blurred vision, double vision or a red or bloodshot eye may occur, and will usually clear after a few days. Full recovery can take four-to-six weeks.[89] Patients are usually advised to avoid getting water in the eye during the first week after surgery, and to avoid swimming for two-to-three weeks as a conservative approach, to minimise risk of bacterial infection.[7] Most patients can return to normal activities the day after phacoemulsification surgery.[90] Depending on the procedure, patients should avoid driving for at least 24 hours after the surgery, largely due to effects from the anaesthesia, possible swelling affecting focus, and pupil dilation causing excessive glare. At the first post-operative check, the surgeon will usually assess whether vision is suitable for driving.[90]
With small-incision self-sealing wounds used with phacoemulsification, some of the post-operative restrictions common with intracapsular and extracapsular procedures are not relevant. Restrictions against lifting and bending were intended to reduce the risk of the wound opening, because straining increases intraocular pressure. With a self-sealing tunnel incision, however, higher pressure closes the wound more tightly. Routine use of a shield is not usually required because inadvertent finger pressure on the eye should not open a correctly structured incision, which should only open to point pressure.[7] After surgery, to prevent contamination, the eyes should not be rubbed and the use of eye makeup, face cream or lotions should be avoided. Excessive dust, wind, pollen or dirt should also be avoided. Sunglasses should be worn on bright days because the eyes will be more sensitive to bright light for a while.[91]
Topical anti-inflammatory drugs and antibiotics are commonly used in the form of eye-drops to reduce the risk of inflammation and infection. A shield or eye-patch may be prescribed to protect the eye while sleeping. The eye will be checked to ensure the IOL remains in place, and once it has fully stabilised, after about six weeks, vision tests will be used to check whether prescription lenses are needed.[89][2] Where the focal length of the IOL is optimised for distance vision, reading glasses will generally be needed for near focus.[36]
In some cases, the patient is dissatisfied with the optical correction provided by the initial implants, and removal and replacement is necessary. This can occur with more-complex IOL designs when patient expectations do not match with the compromises inherent in these designs, or the patient cannot accommodate the difference in distance and near-focusing of monovision lenses.[30] The patient should not participate in contact or extreme sports or similar activities until cleared to do so by the eye surgeon.[92]
Outcomes
After full recovery, visual acuity depends on the underlying condition of the eye, the choice of IOL, and any long-term complications associated with the surgery. More than 90% of operations are successful in restoring useful vision, with a low complication rate.[93] The World Health Organization (WHO) recommends at least 80% of eyes should have a presenting visual acuity of 6/6 to 6/18 (20/20 to 20/60) after surgery, which is considered a good visual outcome, and that with best correction this should be at least 90%. Acuity of between 6/18 and 6/60 (20/60 to 20/200) is regarded as borderline, and worse than 6/60 (20/200) is considered poor. Borderline or poor visual outcomes are usually due to pre-surgery conditions such as glaucoma, macular disease, and diabetic retinopathy.[94]
A ten-year prospective survey on refractive outcomes from a UK National Health Service (NHS) cataract surgery service from 2006 to 2016 showed a mean difference between the targeted and outcome refraction of −0.07 dioptres, with a standard deviation of 0.67, a mean absolute error of 0.50 dioptres. 88.76% were within one diopter of target refraction and 62.36% within 0.50 dioptres.[95]
In a 2009 study in Sweden, factors that affected predicted refraction error included sex, preoperative visual acuity, and glaucoma, and other eye diseases. Second-eye surgery, macular degeneration, age, and diabetes did not affect predicted outcome. Prediction error decreased with time, which is likely due to the use of improved equipment and techniques, including more-accurate biometry.[96] An American survey of nearly two million bilateral cataract surgery patients published in 2013 found immediate sequential bilateral cataract surgery was statistically associated with worse visual outcomes than for delayed sequential bilateral cataract surgery; the difference was small and may not be clinically relevant.[97]
History
Cataract surgery has a long history in Europe, Asia, and Africa. It is one of the commonest and most-successful procedures in worldwide use. The success is due to improvements in techniques for cataract removal and developments in intraocular lens (IOL) replacement technology, in implantation techniques, and in IOL design, construction, and selection.[98] Surgical techniques that have contributed to this success include microsurgery, viscoelastics, and phacoemulsification.[99]
Couching

Couching is the earliest-documented form of cataract surgery, and one of the oldest surgical procedures. In this technique, the lens is dislodged and pushed aside but not removed from the eye, thus removing the opacity but also the ability to focus. Couching was used for centuries but it has generally poor outcomes and is currently routinely practiced only in remote areas of developing countries.[100][101]
Cataract surgery was first mentioned in the Babylonian code of Hammurabi 1750 BCE.[102] The earliest known depiction of cataract surgery is on a statue from the Fifth Dynasty of Egypt (2467–2457 BCE).[102] According to Francisco J Ascaso et al, a "relief painting from tomb number TT 217 in a worker settlement in Deir el-Medina" shows "the man buried in the tomb, Ipuy ... one of the builders of royal tombs in the renowned Valley of the Kings, circa 1279–1213 BC" as he underwent cataract surgery. Although direct evidence for cataract surgery in ancient Egypt is lacking, the indirect evidence, including surgical instruments that could have been used, show that it was possible. It is assumed that the couching technique was used.[102][103]
Couching was practised in ancient India and subsequently introduced to other countries by the Indian physician Sushruta (c. 6th century BCE),[104] who described it in his work Sushruta Samhita ("Compendium of Sushruta"); the work's Uttaratantra section[lower-alpha 1] describes an operation in which a curved needle was used to push the opaque "phlegmatic matter"[lower-alpha 2] in the eye out of the way of vision. The phlegm was then said to be blown out of the nose. The eye would later be soaked with warm, clarified butter and then bandaged.[105] The removal of cataracts by surgery was introduced into China from India, and flourished in the Sui (581–618 CE) and Tang dynasties (618–907 CE).[106]
The first references to cataract and its treatment in Europe are found in 29 CE in De Medicina, the work of the Latin encyclopedist Aulus Cornelius Celsus, which describes a couching operation.[107] Galen of Pergamon 2nd century CE, a prominent Greek physician, surgeon, and philosopher performed an operation to remove a cataract-affected lens using a needle-shaped instrument.[108][109] Although many 20th-century historians have claimed Galen believed the lens to be in the exact centre of the eye, there is evidence he understood the crystalline lens is located in the anterior aspect of the eye.[110]
The removal of cataracts by couching was a common surgical procedure in Djenné[111] and many other parts of Africa.[112] Couching continued to be used throughout the Middle Ages, and is still used in some parts of Africa and in Yemen.[113][101] Couching is an ineffective and dangerous method of cataract therapy, and often results in patients remaining blind or with only partially restored vision.[113] It has mostly been replaced by extracapsular cataract surgery, including phacoemulsification.[114]
The lens can also be removed by suction through a hollow instrument. Bronze oral-suction instruments that seem to have been used for this method of cataract extraction during the 2nd century CE have been unearthed.[115] Such a procedure was described by the 10th-century Persian physician Muhammad ibn Zakariya al-Razi, who attributed it to Antyllus, a 2nd-century Greek physician. The procedure "required a large incision in the eye, a hollow needle, and an assistant with an extraordinary lung capacity".[116] This suction procedure was also described by the Iraqi ophthalmologist Ammar Al-Mawsili, in his 10th-century work Choice of Eye Diseases.[116] He presented case histories of its use, claiming to have had success with it on a number of patients.[116]: p318 Extracting the lens has the benefit of removing the possibility of the lens migrating back into the field of vision.[117] According to the oculist Al-Shādhili, a later variant of the cataract needle in 14th-century Egypt used a screw to grip the lens. It is not clear how often, if ever, this method was used; other writers, including Abu al-Qasim al-Zahrawi and Al-Shadhili, appear to have been unfamiliar with this procedure or claimed it was ineffective.[116]: p319
Eighteenth and nineteenth centuries


In 1748, Jacques Daviel was the first modern European physician to successfully extract cataracts from the eye, removing the cataract from the capsule through a corneal incision with about a 50% success rate.[98] In 1753, Samuel Sharp performed the first-recorded surgical removal of the entire lens and lens capsule. The lens was removed from the eye through a limbal incision.[98] In America, cataract couching may have been performed in 1611,[118] and cataract extraction was most likely performed by 1776.[119] Cataract extraction by aspiration of lens material through a tube using suction was performed by Philadelphia surgeon Philip Syng Physick in 1815.[120]
King Serfoji II Bhonsle of Thanjavur in India performed cataract surgeries in the early 1800s; these are documented in manuscripts at the Saraswathi Mahal Library.[121]
In 1884, Carl Koller, an ophthalmologist from Vienna, first used a cocaine solution on the cornea, as a local anaesthetic. The news of his discovery spread rapidly, but was not without controversy.[122][123]
Early-to-mid 20th century
At the beginning of the 20th century, the standard procedure was intracapsular cataract extraction (ICCE). The work of Henry Smith, who developed a safe, fast way to remove the lens within its capsule by external manipulation, was influential. The capsule forceps, the discovery of enzymatic zonulysis by Joaquin Barraquer in 1957, and the introduction of cryoextraction of the lens by T. Krawicz and Charles Kelman in 1961 continued the development of ICCE.[7] Inracapsular cryoextraction using a liquid-nitrogen-cooled probe tip to freeze the encapsulated lens to the probe was the favoured form of cataract extraction from the late 1960s to the early 1980s.[24][124]
In 1949, Harold Ridley introduced the concept of implantation of the intraocular lens (IOL) which made more-efficient and comfortable visual rehabilitation possible after cataract surgery.[98]
Artificial IOLs are used to replace the eye's natural lens, which is removed during cataract surgery. These lenses increased in popularity since the 1960s. In 1981, the first U.S. Food and Drug Administration (FDA) approval for this type of product was issued. The development of IOLs was an innovation; patients previously did not have their natural lens replaced and had to wear very thick glasses or a special type of contact lens. IOLs can be used to correct other vision problems, such as toric lenses for correcting astigmatism.[35] IOLs can be classified as monofocal, toric, and multifocal lenses.[2]
Also in the 1960s, the development of ultrasound A-scan biometry was a major advance in ocular biometry that contributed to accurate prediction of implant refractive strength.[125]
In 1967, Charles Kelman introduced phacoemulsification, which uses ultrasonic energy to emulsify the nucleus of the crystalline lens to remove the cataracts by aspiration without a large incision. This method of surgery decreased the need for an extended hospital stay and made out-patient surgery the standard. Patients who undergo cataract surgery rarely complain of pain or discomfort during the procedure, although those who have topical anaesthesia rather than peribulbar block anaesthesia may experience some discomfort.[126]
Late 20th century
Ophthalmic viscosurgical devices (OVDs) were introduced in 1972; OVDs facilitated the procedure and improved safety. An OVD is a viscoelastic solution, a gel-like substance used to maintain the shape of the eye at reduced pressure and to protect the inside structure and tissues of the eye without interfering with the operation.[98]
In 1980, Colvard made the cataract incision in the sclera, which limited induced astigmatism.[63] In the early 1980s, Danièle Aron-Rosa and colleagues introduced the neodymium-doped yttrium aluminum garnet laser (Nd:YAG laser) for posterior capsulotomy.[7] Thomas Mazzocco developed the first foldable IOL and implanted it in 1985. Graham Barrett and associates pioneered the use of silicone, acrylics, and hydrogel lenses.[7]
According to Cionni et al (2006), Kimiya Shimizu began removing cataracts using topical anaesthesia in the late 1980s,[7] though Davis (2016) attributes the introduction of topical anaesthetics to Fischman in 1993.[98] In 1987, Blumenthal and Moissiev described the use of a reduced incision size for ECCE. They used a 6.5 to 7 mm (0.26 to 0.28 in) straight scleral tunnel incision 2 mm (0.079 in) behind the limbus with two side ports.[63]
In 1989, McFarland introduced a self-sealing incision architecture; in 1990, Pallin described a chevron-shaped incision that induces the least astigmatism, and in 1991, Singer described the frown incision, in which the ends curve away from the limbus and produces less astigmatism but more than the chevron.[63] Toric IOLs were introduced in 1992 and are used worldwide to correct corneal astigmatism during cataract surgery.[35][98] They have been FDA approved since 1998.[33] Also in the late 1990s, optical biometry based on partial coherence infrared interferometry was introduced. This technique improves visual resolution, offers much greater precision, and is much quicker and more comfortable than ultrasound.[125]
21st century
According to surveys of members of the American Society of Cataract and Refractive Surgery, approximately 2.85 million cataract procedures were performed in the United States during 2004 and 2.79 million in 2005.[127] In 2009, Kosakarn described a method for manual fragmentation of the lens called double-nylon loop. In this technique, the lens is divided into three pieces for extraction, allowing a smaller, sutureless incision of 4.0 to 5.0 mm (0.16 to 0.20 in), and implantation of a foldable IOL. This technique used less expensive instruments and is suitable for use in developing countries.[63]
As of 2013, instruments that use infrared swept-source optical coherence tomography (SS-OCT), a non-invasive, high-speed method that can penetrate dense cataracts and collects thousands of scans per second to generate high-resolution, two-or-three-dimensional data, were avaialble.[125] As of 2021, approximately four million cataract procedures take place annually in the U.S. and nearly 28 million worldwide, a large proportion in India. That is about 75,000 procedures per day globally.[128]
Regional practice and statistics
United Kingdom
In the UK, the practice of NHS healthcare providers referring people with cataracts to surgery widely varied as of 2017; many of the providers were only referring people with moderate or severe vision loss, and often with delays.[129] This practise occurred despite guidance the NHS Executive issued in 2000 urging providers to standardize care, streamline the process, and increase the number of cataract surgeries performed to meet the needs of the aging population.[130] In 2019, the national ophthalmology outcomes audit found five NHS trusts had complication rates of between 1.5% and 2.1%, but since the first national cataract audit in 2010, there had been a 38% reduction in posterior capsule rupture complications.[131]
Asia
South Asia has the highest global age-standardized prevalence of moderate-to-severe visual impairment (17.5%) and mild vision impairment (12.2%). The distribution of ophthalmologists ranges from more than 114 per million of population in Japan to none in Micronesia. Cataract has traditionally been a major cause of blindness in less-developed countries in the region, and although the volume and quality of cataract surgeries have improved, the cataract surgery rate (CSR) remains low for some of these countries.[132]
China
Cataracts are common in China; the overall prevalence in 2022 in Chinese people over 50 years old was 27.45% This was split by environment; the rural prevalence was 28.79% and the urban prevalence was 26.66%. Prevalence of cataract considerably varies by age group; from ages 50 to 59 it is 7.88%, from 60 to 69 it is 24.94%, from 70 to 79 it is 51.74%, and in those over 80 years old it is 78.43%. The overall cataract-surgery coverage rate was 9.19%. The prevalence of cataract and cataract surgical coverage also significantly varies by region.[133]
India
India has raised its cataract-surgical rate from just over 700 operations per million people per year in 1981 to 6,000 per million per year in 2011, which is much closer to the estimated rate of 8,000 to 8,700 per million per year needed to eliminate blindness due to cataracts in India. This is partly a combined result of increased efficiency due to improved surgical techniques with surgeons able to perform twenty operations per day, application of day-case surgery, improvements in operating theatre design, and efficient teamwork with sufficient staff.[134]
In India, people apply for surgery in large numbers. This has been achieved by creating a demand for cataract surgery in the community. Social marketing methods were used to increase awareness of cataracts and that they are a common, age-related problem that can be treated effectively and affordably. The non-governmental organization (NGO) sector and Indian ophthalmologists have developed methods to deal with local problems, including outreach camps to find those needing surgery, counsellors to explain the system, locally manufactured equipment and consumables, and a tiered pricing structure using subsidies where appropriate.[134]
There have been occasional incidents in which patients have been infected and developed endophthalmitis on the same day at some hospitals associated with eye camps in India. Journalists have reported blame being placed on the surgeons, the hospital administration, and other persons, but have not reported on those responsible for sterilizing the surgical instruments and operating theatres involved, whether all infections involved the same micro-organisms, the same theatres, or the same staff. One investigation found bacteria known to be associated with endophthalmia in the theatre and in the eyes of affected patients, and it was claimed the hospital had not followed the required protocol for infection control, but the investigation was ongoing. Several instances of surgeons performing more operations per day than officially allowed have been reported, but the effects upon sterility of equipment or plausible infection pathways have not been reported.[135]
In 2022, the digital news publication Scroll.in requested information from the central health ministry on the numbers of patients who had contracted infections; they were informed since 2006, 469 people had either been blinded in one eye or had their vision seriously affected after undergoing surgery at eye camps. Further inquiries found the number was at least 519 but the total number of surgeries for that period was not mentioned.[135] because large numbers of eye surgeries are performed in India—as of 2017, India is claimed to be performing about 6.5 million cataract surgeries per year, more than the US, Europe and China together—this is a very small percentage.[136]
Africa

Cataracts are the main cause of blindness in Africa, and affect approximately half of the estimated seven million blind people on the continent, a number that is expected to increase with population growth by about 600,000 people per year. The cataract-surgery rate is about 500 per million per year as of 2005. Progress on gathering information on epidemiology, distribution and impact of cataracts within Africa has been made but significant problems and barriers remain.[137]
These barriers relate to awareness, acceptance, and cost; some studies reported community and family dynamics as barriers. The cataract-surgical rate was lower in females in the majority of studies. The higher cataract-surgery coverage found in some settings in South Africa, Libya, and Kenya suggest many barriers to surgery can be overcome.[138]
According to the International Agency for the Prevention of Blindness, some sub-Saharan African countries have about one ophthalmologist per million people, and according to the National Center for Biotechnology Information, the percentage of adults above the age of 50 in western sub-Saharan Africa who are blind because of cataracts is about 6%—the highest rate in the world.[139]
A mathematical model using survey data from sub-Saharan Africa showed the incidence of cataracts varies significantly across the continent, and therefore the required rate of surgery to maintain a visual acuity level of 6/18 (20/60) varies significantly from about 1,200 to about 4,500 surgeries per year per million people. This variation may relate to genetic or cultural differences, and to life expectancy.[140]
Nigeria
In 2011, 0.78% of the population of Nigeria were blind; more than 43% of these were blind from cataracts, and another 9% from aphakia and complications from couching performed by itinerant practitioners. There are about 2.8 ophthalmologists per million population in Nigeria but the cataract-surgery rate is only 300 per million per year, compared with the WHO recommendation of 3,000 per million per year. Reasons cited for this situation include inadequate blindness prevention programs, lack of political will, shortage of funding, and failure of the government to take responsibility for training and services. Teaching hospitals do not have enough patient-surgical load to support training.[101]
South Africa
In South Africa, facilities vary from government hospitals, where subsidised operations for the disadvantaged may be charged at rates that cover the consumables, to private clinics in which up-to-date equipment is used and patients are charged at premium rates. Waiting times in government hospitals may be up to two years whereas those at private clinics are much shorter. Some hospitals use a system in which two patients are operated upon for cataracts in the theatre at the same time, increasing the efficiency of facilities.[141] Some charitable organisations in the country provide pro bono cataract surgery in rural areas using mobile clinics.
As of 2023, the cataract-surgery rate in South Africa is less than half of the estimated requirement of at least 2,000 per million population per year needed to eliminate cataract blindness.[142][143] In 2011, Lecuona and Cook identified an inadequate level of human resources in the public sector to provide care for the indigent population.[143] The main barrier to increasing South Africa's rate of cataract surgery is inadequate surgery capacity. A higher annual rate of cataract surgeries by individual surgeons would improve cost effectiveness and personal skills, and also contribute towards an overall reduction of risk.[143]
Latin America

A four-year longitudinal study of 19 Latin-American countries published in 2010 showed most of the countries increased their surgery rates over that period, with increases of up to 186%, but still failed to provide adequate surgical coverage. There is a good correlation between gross national income per capita and cataract-surgery rate in the countries involved in the study.[144]
In a study published in 2014, the weighted-mean regional surgery rate was found to have increased by 70% from 2005 to 2012, from 1,562 to 2,672 cataract surgeries per million inhabitants. The weighted mean number of ophthalmologists per million inhabitants in the region is approximately 62. Cataract-surgery coverage widely varies in Latin America from 15% in El Salvador to 77% in Uruguay. Barriers cited include cost of surgery and lack of awareness of the availability of surgery. The number iof available ophthalmologists appear to be adequate but the number of those who practise eye surgery is unknown.[145]
In a study published in 2009, the prevalence of cataract blindness in people 50 years and older ranged from 0.5% in Buenos Aires to 2.3% in parts of Guatemala. Poor vision due to cataracts ranged from 0.9% in Buenos Aires to 10.7% in parts of Peru. Cataract-surgical coverage ranged from good in parts of Brazil to poor in Paraguay, Peru, and Guatemala. Visual outcome after cataract surgery was close to conformity with WHO guidelines in Buenos Aires, where more than 80% of post-surgery eyes had visual acuity of 6/18 (20/60) or better, but ranged between 60% and 79% in most of the other regions, and was less than 60% in Guatemala and Peru.[146]
Social and economic relevance
The cost of cataract surgery depends on the type of procedure, whether it is provided privately or by a government hospital, whether it is provided by out-patient (day care) or in-patient surgery, and on the economic status of people in the region. Because of the high cost of the equipment, phaco surgery is generally more expensive than ECCE and MSICS.[6] Visual outcomes are variable; they depend upon the underlying condition of the eyes, and the surgical techniques and lens implants used. Regional variations exist due to quality and availability of care.
The restoration of functional vision or improvement in vision possible in most cases has a large social and economic impact; the person may be able to return to paid work or continue working, and may not become dependent upon on support from their family or the wider society. Studies show a sustained improvement to quality of life, financial situation, physical well-being, and mental health. Cataract surgery is one of the most-cost-effective health interventions; its economic benefits considerably exceed the cost of treatment.[147][148]
The 1998 World Health Report estimated 19.34 million people were bilaterally blind due to age-related cataracts, and that cataracts were responsible for 43% of all cases of blindness. This number and proportion were expected to increase due to population growth, and increased life expectancy approximately doubling the number of people older than 60 years. The global increase in blindness from cataract is estimated to be at least five million per year; a figure of 1,000 new cases per million population per year is used for planning purposes. The average outcomes of cataract surgery are improving, and consequently, surgery is being indicated at an earlier stage in cataract progression, increasing the number of operable cases. To reduce the backlog of patients, it is necessary to operate on more eyes per year than the new cases alone.[149]
The rate of surgeries in economically developed countries as of 1998 was about 4,000 to 6,000 per million population per year, which was sufficient to meet demand. India raised the cataract surgery rate (CSR) to over 3,000 but this does not seem to be sufficient to reduce the backlog. Middle-income countries of Latin America and Asia have CSRs of between 500 and 2,000 per million per year, and China, most of Africa, and poor countries of Asia had rates of less than 500. In India and South East Asia, the rate required to keep up with the increase is at least 3,000 per million population per year, and in Africa and other parts of the world with smaller percentages of older people, a rate of 2,000 may be sufficient in the short term.[149]
Vision 2020: The Right to Sight, a global initiative of the International Agency for the Prevention of Blindness (IAPB), was intended to reduce or eliminate the main causes of avoidable blindness worldwide by 2020. Programs instituted under Vision 2020 facilitated the planning, development, and implementation of sustainable national eye-care programs, including technical support and advocacy.[150] The IAPB and WHO launched the program on 18 February 1999.[151][152]
The Vision 2020 initiative succeeded in bringing avoidable blindness to the global health agenda. The causes have not been eliminated but there have been significant changes to the distribution of the causes, which have been attributed to global demographic shifts. Remaining challenges to management of avoidable blindness include population size, gender disparities in access to eye-care, and the availability of a professional workforce.[152]
It has been estimated there were 43.3 million blind people in 2020, and 295 million with moderate and severe visual impairment (MSVI), 55% of whom were female. The age-standardised global prevalence in blindness decreased by 28.5% between 1990 and 2020, but the age-standardised prevalence of MSVI increased by 2.5%. Cataract remained the global leading cause of blindness in 2020.[152]
Special populations
Congenital cataracts

Congenital cataracts, a lens opacity that is present at birth, occur in a broad range of severity; some lens opacities do not progress and are visually insignificant while others can produce profound visual impairment. Congenital cataracts may be unilateral or bilateral. They can be classified by morphology, presumed or defined genetic cause, presence of specific metabolic disorders, or associated ocular anomalies or systemic findings.[3]
In general, there is greater urgency to remove dense cataracts from very young children because of the risk of amblyopia. For optimal visual development in newborns and young infants, a visually significant unilateral congenital cataract should be detected and removed before the child is six weeks old, and visually significant bilateral congenital cataracts should be removed before 10 weeks.[3] Congenital cataracts that are too small to affect vision will not be removed or treated, but may be monitored by an ophthalmologist throughout the patient's life. Commonly, a patient with small congenital cataracts that do not affect vision will be affected later in life, though this will take decades to occur.[153]
As of 2015[update], the standard of care for pediatric cataract surgery for children older than two years is primary posterior intraocular lens (IOL) implantation. Primary IOL implantation before age seven months is considered to have no advantages over aphakia.[154] According to a 2015 study, primary IOL implantation in the seven-months-to-two-years age groups should be considered in children who require cataract surgery.[154] Research into the possibility of regeneration of infant lenses from lens epithelial cells showed interesting results in a small trial study reported in 2016.[155][156]
Developing world
The capital equipment for phacoemulsification is expensive and requires expert maintenance, and the consumables are also expensive. Quality of outcomes is not sufficiently better than those for manual small incision cataract surgery (MSICS) to justify the difference in cost in a developing world environment.[6]
Higher risk for operations on separate occasions
Most patients have bilateral cataracts; although surgery in one eye can restore functional vision, second-eye surgery has many advantages so most patients undergo surgery in each eye on separate days. Operating on both eyes on the same day as separate procedures is known as immediately sequential bilateral cataract surgery; this can decrease the number of hospital visits, reducing risk of contagion in an epidemic. Immediately sequential bilateral cataract surgery also has significant cost savings, and faster visual rehabilitation and neuroadaptation.[Note 12] Another indication is significant cataracts in both eyes of patients for whom two rounds of anaesthesia and surgery would be unsuitable. The risk of simultaneous bilateral complications is low.[157][158]
See also
- Medicine:Eye surgery – Surgery performed on the eye or its adnexa
- Medicine:IOLVIP – Intraocular lens system to compensate for macular degeneration
- Medicine:Ophthalmology – Field of medicine treating eye disorders
Notes
- ↑ Intraoperative aberrometry: A tool to take aphakic and pseudophakic refractive measurements during surgery to help optimise IOL power selection and placement.
- ↑ Posterior capsule rupture: Unintended tearing of the posterior membrane of the lens capsule, which can allow migration of the vireous into the anterior chamber.
- ↑ Bridle suture: A suture passing through the superior rectus muscle of the eye, used to rotate the eyeball downwards in eye surgery.
- ↑ Hydroexpression: Method of removing the lens from the capsule and anterior chamber by carrying it out in a flow of saline solution.
- ↑ Viscoexpression: Method of removing the lens from the capsule and anterior chamber by carrying it out in a flow of viscoelastic material.
- ↑ Zonular dehiscence: Breaking of the fibrous strands (zonules) connecting the crystalline lens to the ciliary body.
- ↑ Dropped nucleus: A cataract nucleus which has fallen through into the vitreous chamber.
- ↑ Nanophthalmic: Exceptionally small eyes.
- ↑ Exchange: The IOL is replaced with another of the same model.
- ↑ Remove: The IOL is removed and replaced with a different model lens or no replacement lens is implanted.
- ↑ Reposition: The IOL is surgically moved to another location or rotated.
- ↑ Neuroadaptation: Changes in the brain which accommodate the presence of a new substance or condition, such as the admission of more blue light after removal of a yellow tinted cataract, or the inability to adjust the focus of an IOL by the ciliary muscles.
References
- ↑ 1.0 1.1 "Cataracts". National Eye Institute. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/cataracts.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 Moshirfar, Majid; Milner, Dallin; Patel, Bhupendra C. (June 21, 2022). Cataract Surgery. National Center for Biotechnology Information. PMID 32644679. https://www.ncbi.nlm.nih.gov/books/NBK559253/. Retrieved 8 February 2023.
- ↑ 3.0 3.1 3.2 Basic and clinical science course (2011–2012). Pediatric ophthalmology and Strabismus. American Academy of Ophthalmology. ISBN 978-1615251131.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 "Facts About Cataract". September 2009. http://www.nei.nih.gov/health/cataract/cataract_facts.
- ↑ "Cataract surgery". https://www.mayoclinic.org/tests-procedures/cataract-surgery/about/pac-20384765.
- ↑ 6.0 6.1 6.2 6.3 6.4 Haldipurkar, S.S.; Shikari, Hasanain T.; Gokhale, Vishwanath (2009). "Wound construction in manual small incision cataract surgery". Indian Journal of Ophthalmology 57 (1): 9–13. doi:10.4103/0301-4738.44491. ISSN 0301-4738. PMID 19075401.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 7.20 7.21 7.22 7.23 7.24 Cionni, Robert J.; Snyder, Michael E.; Osher, Robert H. (2006). "6: Cataract surgery". in Tasman, William. Duane's Opthalmology. 6. Lippincott, Williams and Wilkins. http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v6/v6c006.html. Retrieved 16 February 2023.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Gurnani, B.; Kaur, K. (6 December 2022). "Manual Small Incision Cataract Surgery.". StatPearls [Internet] (Treasure Island, FL: StatPearls Publishing). PMID 35881728. https://www.ncbi.nlm.nih.gov/books/NBK582123/. Retrieved 2 March 2023.
- ↑ 9.0 9.1 Pandey, S.K. (2005). Pediatric cataract surgery techniques, complications, and management. Philadelphia: Lippincott Williams & Wilkins. p. 20. ISBN 978-0781743075. https://books.google.com/books?id=gLJZDD2igCMC&pg=PA20.
- ↑ Allen, D.; Vasavada, A. (July 2006). "Cataract and surgery for cataract". BMJ 333 (7559): 128–132. doi:10.1136/bmj.333.7559.128. PMID 16840470.
- ↑ Gimbel, H.V.; Dardzhikova, A.A. (January 2011). "Consequences of waiting for cataract surgery". Current Opinion in Ophthalmology 22 (1): 28–30. doi:10.1097/icu.0b013e328341425d. PMID 21076306.
- ↑ 12.0 12.1 12.2 "Priority eye diseases". https://www.who.int/blindness/causes/priority/en/index1.html.
- ↑ Lamoureux, E.L.; Fenwick, E.; Pesudovs, K.; Tan, D. (January 2011). "The impact of cataract surgery on quality of life". Current Opinion in Ophthalmology 22 (1): 19–27. doi:10.1097/icu.0b013e3283414284. PMID 21088580.
- ↑ 14.0 14.1 Rao, G.N.; Khanna, R.; Payal, A. (January 2011). "The global burden of cataract". Current Opinion in Ophthalmology 22 (1): 4–9. doi:10.1097/icu.0b013e3283414fc8. PMID 21107260.
- ↑ "Visual impairment and blindness Fact Sheet N°282". August 2014. https://www.who.int/mediacentre/factsheets/fs282/en/.
- ↑ Global Data on Visual Impairments 2010. WHO. 2012. p. 6. https://www.who.int/blindness/GLOBALDATAFINALforweb.pdf?ua=1.
- ↑ 17.0 17.1 "Cataract Data and Statistics: National Eye Institute". https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/cataract-data-and-statistics.
- ↑ 18.0 18.1 Lambert, S.R.; Aakalu, V.K.; Hutchinson, A.K.; Pineles, S.L.; Galvin, J.A.; Heidary, G.; Binenbaum, G.; VanderVeen, D.K. (October 2019). "Intraocular Lens Implantation during Early Childhood: A Report by the American Academy of Ophthalmology". Ophthalmology 126 (10): 1454–1461. doi:10.1016/j.ophtha.2019.05.009. PMID 31230794. https://pubmed.ncbi.nlm.nih.gov/31230794/. Retrieved 2023-02-27.
- ↑ Yorston, D. (2001). "Intraocular Lens (IOL) Implants in Children". Community Eye Health 14 (40): 57–8. PMID 17491933.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 Agarwal, Ashvin (March 2019). "When and How to Convert to ECCE: Extracapsular cataract extraction remains a useful plan B.". https://crstoday.com/articles/2019-mar/when-and-how-to-convert-to-ecce.
- ↑ Lawrence, D.; Fedorowicz, Z.; van Zuuren, E.J. (November 2015). "Day care versus in-patient surgery for age-related cataract". The Cochrane Database of Systematic Reviews 2015 (11): CD004242. doi:10.1002/14651858.CD004242.pub5. PMID 26524611.
- ↑ Alió, J.L.; Abdou, A.A.; Puente, A.A.; Zato, M.A.; Nagy, Z. (June 2014). "Femtosecond laser cataract surgery: updates on technologies and outcomes". Journal of Refractive Surgery 30 (6): 420–427. doi:10.3928/1081597x-20140516-01. PMID 24972409.
- ↑ Popovic, M.; Campos-Möller, X; Schlenker, M.B.; Ahmed, I.I. (October 2016). "Efficacy and Safety of Femtosecond Laser-Assisted Cataract Surgery Compared with Manual Cataract Surgery: A Meta-Analysis of 14 567 Eyes". Ophthalmology 123 (10): 2113–2126. doi:10.1016/j.ophtha.2016.07.005. PMID 27538796.
- ↑ 24.0 24.1 Toczolowski, J. (July 1993). "Thirty years of cryoophthalmology". Ann. Ophthalmol. 25 (7): 254–6. PMID 8363292. https://pubmed.ncbi.nlm.nih.gov/8363292/. Retrieved 2023-02-12.
- ↑ Kim, Y.J.; Ha, S.J. (2013). "Intracapsular Lens Extraction for the Treatment of Pupillary Block Glaucoma Associated with Anterior Subluxation of the Crystalline Lens.". Case Rep Ophthalmol 4 (3): 257–264. doi:10.1159/000356530. PMID 24348413.
- ↑ Barsam, Allon; Allan, Bruce (17 July 2014). "Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia". Cochrane Database of Systematic Reviews (6): CD007679. doi:10.1002/14651858.cd007679.pub4. ISSN 1465-1858. PMID 24937100.
- ↑ 27.0 27.1 Scholtz, Sibylle (January 2007). "History of Ophthalmic Viscosurgical Devices". Cataract & Refractive Surgery Today Europe. https://crstodayeurope.com/articles/2007-jan/0107_06-php/.
- ↑ 28.0 28.1 28.2 Sridhar, U; Tripathy, K. (22 August 2022). "Monofocal Intraocular Lenses.". StatPearls [Internet] (Treasure Island, FL: StatPearls Publishing). PMID 35593809. https://www.ncbi.nlm.nih.gov/books/NBK580484/. Retrieved 8 February 2023.
- ↑ 29.0 29.1 Mehta, R.; Aref, A.A. (November 2019). "Intraocular Lens Implantation In The Ciliary Sulcus: Challenges And Risks". Clin. Ophthalmol. 27 (13): 2317–2323. doi:10.2147/OPTH.S205148. PMID 31819356.
- ↑ 30.0 30.1 Grayson, Douglas (4 October 2011). "The Ins and Outs of Lens Explantation". https://www.reviewofophthalmology.com/article/the-ins-and-outs-of-lens-explantation.
- ↑ Salerno, Liberdade C.; Tiveron, Jr., Mauro C.; Alió, Jorge L. (2017). "Multifocal intraocular lenses: Types, outcomes, complications and how to solve them.". Taiwan Journal of Ophthalmology 7 (4): 179–184. doi:10.4103/tjo.tjo_19_17. PMID 29296549.
- ↑ MacRae, Scott. "Crystalens: The First Accommodating Intraocular Lens Implant". University of Rochester Flaum Eye Institute. https://www.urmc.rochester.edu/eye-institute/lasik/procedures/crystalens-iol.aspx.
- ↑ 33.0 33.1 Ramappa, Muralidhar; Singh, Vivek Mahendrapratap; Murthy, SomasheilaI; Rostov, AudreyTalley (2022). "Toric intraocular lenses: Expanding indications and preoperative and surgical considerations to improve outcomes" (in en). Indian Journal of Ophthalmology 70 (1): 10–23. doi:10.4103/ijo.IJO_1785_21. ISSN 0301-4738. PMID 34937203.
- ↑ 34.0 34.1 Roach, Linda (September 2013). "Intraoperative Wavefront Aberrometry: Wave of the Future?". EyeNet Magazine (American Academy of Ophthalmology). https://www.aao.org/eyenet/article/intraoperative-wavefront-aberrometry-wave-of-futur.
- ↑ 35.0 35.1 35.2 Singh, Vivek Mahendrapratap; Ramappa, Muralidhar; Murthy, Somasheila; Rostov, Audrey Talley (January 2022). "Toric intraocular lenses: Expanding indications and preoperative and surgical considerations to improve outcomes". Indian J Ophthalmol 70 (1): 10–23. doi:10.4103/ijo.IJO_1785_21. PMID 34937203.
- ↑ 36.0 36.1 Yetman, Daniel (13 January 2023). "Do You Need Glasses After Cataract Surgery?". in Lloyd III, William C.. https://www.healthline.com/health/eye-health/glasses-after-cataract-surgery.
- ↑ Roach, Linda (November–December 2010). "How to Choose an Aspheric Intraocular Lens". EyeNet Magazine (American Academy of Ophthalmology). https://www.aao.org/eyenet/article/how-to-choose-aspheric-intraocular-lens.
- ↑ Downie, L.E.; Busija, L.; Keller, P.R. (May 2018). "Blue-light filtering intraocular lenses (IOLs) for protecting macular health". The Cochrane Database of Systematic Reviews 2018 (5): CD011977. doi:10.1002/14651858.CD011977.pub2. PMID 29786830.
- ↑ 39.0 39.1 Hayashi, K.; Hayashi, H. (2006). "Visual function in patients with yellow tinted intraocular lenses compared with vision in patients with non-tinted intraocular lenses". British Journal of Ophthalmology 90 (8): 1019–1023. doi:10.1136/bjo.2006.090712. PMID 16597662.
- ↑ "FDA Approves RxSight's Light Adjustable Lens, First IOL To Enable Refractive Correction After Cataract Surgery". University of California San Francisco. 27 November 2017. https://innovation.ucsf.edu/news/fda-approves-rxsights-light-adjustable-lens-first-iol-enable-refractive-correction-after. Retrieved 21 February 2023.
- ↑ Jain, Sneha; Patel, Alpa S.; Tripathy, Koushik; DelMonte, Derek W.; Baartman, Brandon (3 October 2022). "Light Adjustable Intraocular lenses". in DelMonte, Derek W.. EyeWiki. American Academy of Opthalmology. https://eyewiki.aao.org/Light_Adjustable_Intraocular_lenses.
- ↑ Portelinha, Joana; Ferreira, Tiago Luís do Carmo Bravo; Reddy, Vandana; Shafer, Brian (8 January 2023). "Special Cases: Secondary Piggy-Back Lenses". in Shafer, Brian. American Academy of Ophthalmology. https://eyewiki.aao.org/Special_Cases:_Secondary_Piggy-Back_Lenses.
- ↑ Hasan, Sumaiya; Tripathy, K. (22 August 2022). "Phakic Intraocular Lens Myopia". StatPearls [Internet] (Treasure Island, FL: StatPearls Publishing). PMID 32809598. https://www.ncbi.nlm.nih.gov/books/NBK560763/. Retrieved 21 February 2023.
- ↑ 44.0 44.1 44.2 44.3 44.4 44.5 Goldsberry, Dennis H. (May 2012). "Achieving Better Outcomes Using Free Online Post-LASIK IOL Calculators". CRSTEurope. https://crstodayeurope.com/articles/2012-may/achieving-better-outcomes-using-free-online-post-lasik-iol-calculators/.
- ↑ Straub, Laura. "SRK/T Formula: A Review: A combination of linear regression method with a theoretical eye model.". CRSTEurope. https://crstodayeurope.com/articles/2012-may/srkt-formula-a-review/.
- ↑ Vicchrilli, Sue; Glasser, David B.; McNett, Cherie; Burke, Mara Pearse; Repka, Michael X. (October 2018). "Premium IOLs—A Legal and Ethical Guide to Billing Medicare Beneficiaries". EyeNet Magazine. https://www.aao.org/eyenet/article/premium-iols-a-legal-and-ethical-guide.
- ↑ Liaska, A; Papaconstantinou, D; Georgalis, I; Koutsandrea, C; Theodosiadis, P; Chatzistefanou, K. (July 2014). "Phaco-trabeculectomy in controlled, advanced, open-angle glaucoma and cataract: Parallel, randomized clinical study of efficacy and safety.". Semin Ophthalmol. 29 (4): 226–35. doi:10.3109/08820538.2014.880491. PMID 24654699.
- ↑ Akman, A; Yilmaz, G; Oto, S; Akova, YA (September 2004). "Comparison of various pupil dilatation methods for phacoemulsification in eyes with a small pupil secondary to pseudoexfoliation.". Ophthalmology 111 (9): 1693–8. doi:10.1016/j.ophtha.2004.02.008. PMID 15350324.
- ↑ Rishi, P.; Sharma, T.; Rishi, E.; Chaudhary, S.P. (January–April 2009). "Combined scleral buckling and phacoemulsification". Oman J Ophthalmol 2 (1): 15–8. doi:10.4103/0974-620X.48416. PMID 21234218.
- ↑ Charters, Linda (15 June 2006). "Anticipation is key to managing intra-operative floppy iris syndrome". Ophthalmology Times. http://www.ophthalmologytimes.com/ophthalmologytimes/article/articleDetail.jsp?id=339794.
- ↑ Keay, L.; Lindsley, K.; Tielsch, J.; Katz, J.; Schein, O. (January 2019). "Routine preoperative medical testing for cataract surgery". The Cochrane Database of Systematic Reviews 1 (1): CD007293. doi:10.1002/14651858.CD007293.pub4. PMID 30616299.
- ↑ Wier, L.M.; Steiner, C.A.; Owens, P.L. (February 2015). "Surgeries in Hospital-Owned Outpatient Facilities, 2012". HCUP Statistical Brief #188. Rockville, MD: Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb188-Surgeries-Hospital-Outpatient-Facilities-2012.jsp.
- ↑ 53.0 53.1 Ou, Yvonne (5 July 2021). "Side Effects of Laser Iridotomy". University of California, San Francisco. https://www.brightfocus.org/glaucoma/article/side-effects-laser-iridotomy.
- ↑ Miguel, Ana IM; Marques, Sara HM; Cruz, Mário; Aref, Ahmad A.; Silva, André Borges; Tsui, Jonathan C. (25 December 2022). "Laser Peripheral Iridotomy". in Aref, Ahmad A.. Eyewiki. American Academy of Opthalmology. https://eyewiki.aao.org/Laser_Peripheral_Iridotomy.
- ↑ "Cataract surgery". https://www.mayoclinic.org/tests-procedures/cataract-surgery/about/pac-20384765.
- ↑ 56.0 56.1 56.2 56.3 Minakaran, N.; Ezra, D.G.; Allan, B.D. (July 2020). "Topical anaesthesia plus intracameral lidocaine versus topical anaesthesia alone for phacoemulsification cataract surgery in adults". The Cochrane Database of Systematic Reviews 2020 (7): CD005276. doi:10.1002/14651858.cd005276.pub4. PMID 35658539.
- ↑ 57.0 57.1 57.2 57.3 Gurnani, Bharat; Kaur, Kirandeep (6 December 2022). "Phacoemulsification". StatPearls [Internet] (Treasure Island, FL: StatPearls Publishing). PMID 35015444. https://www.ncbi.nlm.nih.gov/books/NBK576419/. Retrieved 20 February 2023.
- ↑ Mohammadpour, M.; Erfanian, R.; Karimi, N. (January 2012). "Capsulorhexis: Pearls and pitfalls". Saudi J Ophthalmol 26 (1): 33–40. doi:10.1016/j.sjopt.2011.10.007. PMID 23960966.
- ↑ Yanoff, Myron; Duker, Jay S. (1 January 2009). Ophthalmology. Elsevier Health Sciences. ISBN 978-0323043328. https://books.google.com/books?id=u43MTFr7-m8C&dq=Hydrodissection&pg=PA461. Retrieved 19 February 2023.
- ↑ Faust, KJ. (Winter 1984). "Hydrodissection of soft nuclei.". J Am Intraocul Implant Soc. 10 (1): 75–7. doi:10.1016/s0146-2776(84)80088-9. PMID 6706823.
- ↑ Patel, Alpa S.; DelMonte, Derek W.; Mohan, Hridya; Christenbury, Joseph (24 September 2022). "Hydro Manoeuvres in Cataract Surgery". in Christenbury, Joseph. American Academy of Ophthalmology. https://eyewiki.aao.org/Hydro_Manoeuvres_in_Cataract_Surgery#cite_note-faust1-1.
- ↑ Mathey, Christoph F.; Kohnen, Thomas B.; Ensikat, Hans-Jürgen; Koch, Hans-Reinhard (January 1994). "Polishing methods for the lens capsule: Histology and scanning electron microscopy". Journal of Cataract & Refractive Surgery 20 (1): 64–69. doi:10.1016/S0886-3350(13)80046-6. PMID 8133483. https://www.sciencedirect.com/science/article/abs/pii/S0886335013800466. Retrieved 2023-02-08.
- ↑ 63.0 63.1 63.2 63.3 63.4 63.5 63.6 Singh, K.; Misbah, A.; Saluja, P.; Singh, A.K. (December 2017). "Review of manual small-incision cataract surgery". Indian J Ophthalmol 65 (12): 1281–1288. doi:10.4103/ijo.IJO_863_17. PMID 29208807.
- ↑ Devgan, Uday (27 January 2019). "Use of an AC maintainer in Cataract Surgery". https://cataractcoach.com/2019/01/27/use-of-an-ac-maintainer-in-cataract-surgery/.
- ↑ Oetting, Thomas. "Capsulorhexis using a cystotome needle during cataract surgery". http://www.medrounds.org/bookstore/ProductDetail.php?product_id=79.
- ↑ Sharma, Bhavana; Abell, Robin G; Arora, Tarun; Antony, Tom; Vajpayee, Rasik B (2019). "Techniques of anterior capsulotomy in cataract surgery". Indian Journal of Ophthalmology 67 (4): 450–460. doi:10.4103/ijo.IJO_1728_18. PMID 30900573.
- ↑ Salmon, John F. (2020). Kanski's Clinical Ophthalmology: A Systematic Approach (9th ed.). Edinburgh. p. 331. ISBN 978-0-7020-7713-5. OCLC 1131846767. https://www.worldcat.org/oclc/1131846767. Retrieved 2023-02-20.
- ↑ Thim, K.; Krag, S.; Corydon, L. (March 1993). "Hydroexpression and viscoexpression of the nucleus through a continuous circular capsulorhexis". J Cataract Refract Surg 19 (2): 209–12. doi:10.1016/s0886-3350(13)80944-3. PMID 8487162. https://pubmed.ncbi.nlm.nih.gov/8487162/. Retrieved 2023-03-01.
- ↑ Varshney, S.; Jhala, L.S. (November 2022). "Hydroexpression - A novel technique to deliver nucleus in small-incision cataract surgery". Indian J Ophthalmol 70 (11): 4066. doi:10.4103/ijo.IJO_1594_22. PMID 36308162.
- ↑ Mohan, S.; John, B.; Rajan, M.; Malkani, H.; Nagalekshmi, S.V.; Singh, S. (June 2017). "Glued intraocular lens implantation for eyes with inadequate capsular support: Analysis of the postoperative visual outcome". Indian J Ophthalmol 65 (6): 472–476. doi:10.4103/ijo.IJO_375_16. PMID 28643711.
- ↑ 71.0 71.1 Wang, Robert C.; Fuller, Dwain G.; Hutton, William S. (2006). "66: Retained Lens Material". in Tasman, William. Duane's Opthalmology. 6. Lippincott, Williams and Wilkins. http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v6/v6c065.html. Retrieved 16 February 2023.
- ↑ Vajpayee, R.B.; Sharma, N.; Dada, T.; Gupta, V.; Kumar, A.; Dada, V.K. (1 June 2001). "Management of posterior capsule tears". Surv Ophthalmol 45 (6): 473–88. doi:10.1016/s0039-6257(01)00195-3. PMID 11425354.
- ↑ Chaturvedi, Vivek; Sabherwal, Ryan; Kim, Leo A.; Pittner, Andrew; Bhagat, Neelakshi; Lim, Jennifer I; Mukkamala, Lekha; Patel, Nimesh (23 June 2022). "Suprachoroidal Hemorrhage". in Patel, Nimesh. Eyewiki. American Academy of Opthalmology. https://eyewiki.aao.org/Suprachoroidal_Hemorrhage.
- ↑ Hilford, D.; Hilford, M.; Mathew, A.; Polkinghorne, P.J. (2009). "Posterior vitreous detachment following cataract surgery". Eye 23 (6): 1388–1392. doi:10.1038/eye.2008.273. PMID 18776863.
- ↑ "Videos: YAG Laser Capsulotomy". https://www.pcli.com/videos/yag-laser-capsulotomy.html.
- ↑ Karahan, Eyyup; Er, Duygu; Kaynak, Suleyman (Summer 2014). "An Overview of Nd:YAG Laser Capsulotomy". Medical Hypothesis, Discovery & Innovation Ophthalmology Journal 3 (2): 45–50. PMID 25738159.
- ↑ 77.0 77.1 Steel, D. (March 2014). "Retinal detachment". BMJ Clinical Evidence 2014. PMID 24807890.
- ↑ Feltgen, N.; Walter, P. (January 2014). "Rhegmatogenous retinal detachment--an ophthalmologic emergency". Deutsches Ärzteblatt International 111 (1–2): 12–21; quiz 22. doi:10.3238/arztebl.2014.0012. PMID 24565273.
- ↑ "Toxic Anterior Segment Syndrome After Cataract Surgery". Centers for Disease Control and Prevention. 29 June 2007. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5625a2.htm. Retrieved 18 April 2013.
- ↑ "Endophthalmitis". Lecturio. https://www.lecturio.com/concepts/endophthalmitis/.
- ↑ Bennett, John E. (8 August 2019). "Endophthalmitis". Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (9th ed.). ISBN 9780323550277.
- ↑ Gokhale, Parag A.; Patterson, Emory (May–June 2007). "Elevated IOP After Cataract Surgery". Glaucoma today (Bryn Mawr Communications, LLC.). https://glaucomatoday.com/articles/2007-may-june/0507_07.html. Retrieved 2023-02-25.
- ↑ Swamy, B.N.; Billson, F.; Martin, F.; Donaldson, C.; Hing, S.; Jamieson, R.; Grigg, J.; Smith, J.E. (December 2007). "Secondary glaucoma after paediatric cataract surgery.". Br J Ophthalmol 91 (12): 1627–30. doi:10.1136/bjo.2007.117887. PMID 17475699.
- ↑ 84.00 84.01 84.02 84.03 84.04 84.05 84.06 84.07 84.08 84.09 84.10 84.11 84.12 84.13 84.14 Masket, Samuel; Rorer, Eva; Stark, Walter; Holladay, Jack T.; MacRae, Scott; Tarver, Michelle E.; Glasser, Adrian; Calogero, Don et al. (January 2017). "Special Report: The American Academy of Ophthalmology Task Force Consensus Statement on Adverse Events with Intraocular Lenses". Ophthalmology 124 (1): 142–144. doi:10.1016/j.ophtha.2016.09.031. PMID 27726961. https://www.aaojournal.org/article/S0161-6420(16)31407-5/fulltext#%20. Retrieved 2023-02-27.
- ↑ Gaton, D.D.; Mimouni, K.; Lusky, M.; Ehrlich, R.; Weinberger, D. (September 2003). "Pupillary block following posterior chamber intraocular lens implantation in adults". Br J Ophthalmol 87 (9): 1109–11. doi:10.1136/bjo.87.9.1109. PMID 12928277.
- ↑ Lim, B.X.; Lim, C.H.; Lim, D.K.; Evans, J.R.; Bunce, C.; Wormald, R. (November 2016). "Prophylactic non-steroidal anti-inflammatory drugs for the prevention of macular oedema after cataract surgery". The Cochrane Database of Systematic Reviews 11 (11): CD006683. doi:10.1002/14651858.CD006683.pub3. PMID 27801522.
- ↑ Zemba, M.; Camburu, G (2017). "Uveitis-Glaucoma-Hyphaema Syndrome. General review". Romanian Journal of Ophthalmology 61 (1): 11–17. doi:10.22336/rjo.2017.3. PMID 29450365.
- ↑ 88.0 88.1 88.2 Stein, Joshua D.; Grossman, Daniel S.; Mundy, Kevin M.; Sugar, Alan; Sloan, Frank A. (2 June 2011). "Severe Adverse Events after Cataract Surgery Among Medicare Beneficiaries". Ophthalmology 118 (9): 1716–1723. doi:10.1016/j.ophtha.2011.02.024. PMID 21640382. PMC 3328508. https://www.aaojournal.org/article/S0161-6420(11)00183-7/fulltext. Retrieved 14 February 2023.
- ↑ 89.0 89.1 "Recovery - Cataract surgery". 15 January 2018. https://www.nhs.uk/conditions/cataract-surgery/recovery/.
- ↑ 90.0 90.1 "How Many Days Rest Are Needed After Cataract Surgery?". 18 May 2022. https://southcaleye.com/cataracts/how-many-days-rest-are-needed-after-cataract-surgery/.
- ↑ Dudek, Lara (15 September 2020). "After Cataract Surgery: Dos and Don'ts". https://www.heartoftexaseye.com/blog/after-cataract-surgery/. Retrieved 22 February 2023.
- ↑ Porter, Daniel (1 August 2022). "When to Resume Exercise After an Eye Surgery or Injury". American Academy of Ophthalmology. https://www.aao.org/eye-health/tips-prevention/resume-exercise-sports-after-eye-surgery-injury.
- ↑ Wong, Tien Yin (5 May 2001). "Effect of increasing age on cataract surgery outcomes in very elderly patients". BMJ 322 (7294): 1104–6. doi:10.1136/bmj.322.7294.1104. PMID 11337443.
- ↑ Hashmi, Farzeen Khalid; Khan, Qazi Assad; Chaudhry, Tanveer Anjum; Ahmad, Khabir (2013). "Visual Outcome of Cataract Surgery". Journal of the College of Physicians and Surgeons Pakistan 23 (6): 448–449. PMID 23763813. https://applications.emro.who.int/imemrf/J_Coll_Physicians_Surg_Pak/J_Coll_Physicians_Surg_Pak_2013_23_6_448_449.pdf.
- ↑ Brogan, K.; Diaper, C.J.; Rotchford, A.P. (2019). "Cataract surgery refractive outcomes: representative standards in a National Health Service setting". British Journal of Ophthalmology 103 (4): 539–543. doi:10.1136/bjophthalmol-2018-312209. PMID 29907629. https://bjo.bmj.com/content/103/4/539.citation-tools. Retrieved 2023-03-03.
- ↑ Kugelberg, Maria; Lundström, Mats (May 2009). "Refractive Outcome After Cataract Surgery". Cataract Surgery. CRST Global: Europe Edition. https://crstodayeurope.com/articles/2009-may/0509_04-php/.
- ↑ Owen, Julia P.; Blazes, Marian; Lacy, Megan; Yanagihara, Ryan T.; Van Gelder, Russell N.; Lee, Aaron Y.; Lee, Cecilia S. (2021). "Refractive Outcomes After Immediate Sequential vs Delayed Sequential Bilateral Cataract Surgery". JAMA Ophthalmol. 139 (8): 876–885. doi:10.1001/jamaophthalmol.2021.2032. PMID 34196667. PMC 8251655. https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2781698. Retrieved 2023-03-03.
- ↑ 98.0 98.1 98.2 98.3 98.4 98.5 98.6 Davis, G. (January–February 2016). "The Evolution of Cataract Surgery". Mo. Med. (Missouri State Medical Association) 113 (1): 58–62. PMID 27039493.
- ↑ "Chapter 5: Microsurgery and Extracapsular Cataract Extraction". pp. 36–44. https://rajswasthya.nic.in/RHSDP%20Training%20Modules/Ophthalmologist/Cataract%20Surgery%20with%20IOL.Pdf/05%20ECCE.pdf.
- ↑ Isawumi, M.A.; Kolawole, O.U.; Hassan, M.B. (June 2013). "Couching techniques for cataract treatment in Osogbo, South west Nigeria". Ghana Medical Journal 47 (2): 64–9. PMID 23966741.
- ↑ 101.0 101.1 101.2 Babalola, O.E. (December 2011). "The peculiar challenges of blindness prevention in Nigeria: a review article.". Afr J Med Med Sci 40 (4): 309–19. PMID 22783680. https://pubmed.ncbi.nlm.nih.gov/22783680/. Retrieved 2023-02-25.
- ↑ 102.0 102.1 102.2 Ascaso, F.J.; Lizana, J.; Cristóbal, J.A. (March 2009). "Cataract surgery in ancient Egypt". Journal of Cataract and Refractive Surgery 35 (3): 607–608. doi:10.1016/j.jcrs.2008.11.052. PMID 19251160.
- ↑ Grzybowski, Andrzej; Ascaso, Francisco J. (November 2014). "Indirect evidence of cataract surgery in ancient Egypt". Journal of Cataract & Refractive Surgery 40 (11): 1944–1945. doi:10.1016/j.jcrs.2014.09.017. PMID 25442904. https://journals.lww.com/jcrs/Fulltext/2014/11000/Indirect_evidence_of_cataract_surgery_in_ancient.44.aspx.
- ↑ Meulenbeld, G.J. (1999–2002). A History of Indian Medical Literature. Groningen: Forsten.
- ↑ Sharma, P.V. (2001). Suśruta-Saṃhitā with English translation of text and Ḍalhaṇa's commentary along with critical notes. Vol ĪI (Kalpasthāna and Uttaratantra). Varanasi, India: Chaukhambha Visvabharati Oriental. pp. 202–204.
- ↑ Deshpande, V. (2000). "Ophthalmic surgery: a chapter in the history of Sino-Indian medical contacts". Bulletin of the School of Oriental and African Studies 63 (3): 370–388. doi:10.1017/S0041977X00008454. See also Deshpande, V. (1999). "Indian influences on early Chinese ophthalmology: glaucoma as a case study". Bulletin of the School of Oriental and African Studies 62 (22): 306–322. doi:10.1017/S0041977X00016724.
- ↑ Aur. Cor. Celsus (1831). "Book 7, chapter 7. On the diseases of the eyes, and those which are cured by manual operations". De Medicina [On Medicine]. De medicina.English & Latin. 2. London: E. Cox. pp. 257–261. https://babel.hathitrust.org/cgi/pt?id=uc1.b3103789&view=1up&seq=9&q1=cataract. Retrieved 2023-02-28. Translated from L.Targa's 1769 edition, includes the original Latin text.
- ↑ Urabe, H. (September 1976). "[Classification of ringworm infections (author's transl)]". Nihon Hifuka Gakkai Zasshi. The Japanese Journal of Dermatology 86 (10): 573–581. PMID 1034789.
- ↑ Magner, L.N. (1992). A History of Medicine. CRC Press. pp. 91.
- ↑ Leffler, C.T.; Hadi, T.M.; Udupa, A.; Schwartz, S.G.; Schwartz, D. (2016). "A medieval fallacy: the crystalline lens in the center of the eye". Clinical Ophthalmology 10 (10): 649–662. doi:10.2147/OPTH.S100708. PMID 27114699.
- ↑ McKissack, P; McKissack, F. (1995). The Royal Kingdoms of Ghana, Mali, and Songhay Life in Medieval Africa. Macmillan. p. 104. ISBN 978-0-8050-4259-7. https://archive.org/details/royalkingdomsofg00patr/page/104.
- ↑ Thompson, E.E. (January 1965). "Primitive African Medical Lore and Witchcraft". Bulletin of the Medical Library Association 53 (1): 80–94. PMID 14223742.
- ↑ 113.0 113.1 "'Couching' for cataracts remains a persistent problem in Yemen". EuroTimes: p. 11. September 2005. http://www.escrs.org/Publications/Eurotimes/05September/pdf/couching.pdf.
- ↑ "Toric Lens Implants | Pacific Cataract and Laser Institute: For Doctors of Optometry". http://odpcli.com/articles/toric-lens-implants1.
- ↑ "Factors influencing the genesis of neurosurgical technology". Neurosurgical Focus 27 (3): E3. September 2009. doi:10.3171/2009.6.FOCUS09117. PMID 19722817.
- ↑ 116.0 116.1 116.2 116.3 Savage-Smith, E. (August 2000). "The practice of surgery in Islamic lands: myth and reality". Social History of Medicine 13 (2): 307–321. doi:10.1093/shm/13.2.307. PMID 14535259.
- ↑ Finger, S. (1994). Origins of Neuroscience: A History of Explorations Into Brain Function. Oxford University Press. p. 70. ISBN 978-0-19-514694-3.
- ↑ "The first cataract surgeons in Latin America: 1611-1830". Clinical Ophthalmology 10: 679–694. 2016. doi:10.2147/OPTH.S105825. PMID 27143845.
- ↑ "The first cataract surgeons in Anglo-America". Survey of Ophthalmology 60 (1): 86–92. 2015. doi:10.1016/j.survophthal.2014.08.002. PMID 25444521.
- ↑ Leffler, C.T.; Letocha, C.E.; Pierson, K.; Schwartz, S.G. (2017). "Aspiration of cataract in 1815 in Philadelphia, Pennsylvania". Digital Journal of Ophthalmology 23 (4): 4–7. doi:10.5693/djo.01.2017.10.001. PMID 29403333.
- ↑ Badrinath, S.S.; Biswas, J.; Badrinath, Vasanthi (2004-10-10). "Prince of Ophthalmology". The Hindu. http://www.hindu.com/thehindu/mag/2004/10/10/stories/2004101000410200.htm.
- ↑ Goerig, M.; Bacon, D; van Zundert, A. (May–June 2012). "Carl Koller, cocaine, and local anesthesia: some less known and forgotten facts". Reg Anesth Pain Med. 37 (3): 318–24. doi:10.1097/AAP.0b013e31825051f3. PMID 22531385. https://pubmed.ncbi.nlm.nih.gov/22531385/.
- ↑ Altman, A.J.; Albert, D.M.; Fournier, G.A. (January–February 1985). "Cocaine's use in ophthalmology: our 100-year heritage". Surv Ophthalmol. 29 (4): 300–6. doi:10.1016/0039-6257(85)90154-7. PMID 3885453. https://pubmed.ncbi.nlm.nih.gov/3885453/.
- ↑ Meadow, Norman B. (15 October 2005). "Cryotherapy: A fall from grace, but not a crash". Ophthalmology Times.
- ↑ 125.0 125.1 125.2 Khoramnia, R.; Auffarth, G.; Łabuz, G.; Pettit, G.; Suryakumar, R. (19 January 2022). "Refractive Outcomes after Cataract Surgery". Diagnostics (Basel) 12 (2): 243. doi:10.3390/diagnostics12020243. PMID 35204334.
- ↑ Pandey, Suresh K.; Milverton, E. John; Maloof, Anthony J. (October 2004). "A tribute to Charles David Kelman MD: ophthalmologist, inventor and pioneer of phacoemulsification surgery". Clinical & Experimental Ophthalmology 32 (5): 529–533. doi:10.1111/j.1442-9071.2004.00887.x. ISSN 1442-6404. PMID 15498067. https://pubmed.ncbi.nlm.nih.gov/15498067/. Retrieved 2022-10-19.
- ↑ "American Academy of Ophthalmology News". 5 April 2006. http://www.aao.org/news/academy_express/20060405.cfm. [yes|permanent dead link|dead link}}]
- ↑ Lindstrom, Richard L. (10 February 2021). "Future of cataract surgery seems promising". occular surgery news (Healio). https://www.healio.com/news/ophthalmology/20210126/future-of-cataract-surgery-seems-promising.
- ↑ "Two thirds of eye units restricting access to cataract surgery". OnMedica. 10 November 2017. https://www.onmedica.com/NewsArticle.aspx?id=d8e682d2-2eb8-4c8f-b0c6-53369c121510.
- ↑ "Action on Cataracts Good Practice Guidance". NHS Executive via the Royal College of Ophthalmologists. January 2000. https://www.rcophth.ac.uk/wp-content/uploads/2015/03/Action-on-cataracts-Jan-2000-dh_4014514.pdf., referenced in "Context: Guideline for Cataracts in adults". NICE. October 2017. https://www.nice.org.uk/guidance/ng77/chapter/Context.
- ↑ "Revealed: The trusts with the highest cataract complication rates". Health Service Journal. 11 October 2019. https://www.hsj.co.uk/quality-and-safety/revealed-the-trusts-with-highest-cataract-complication-rates/7026112.article.
- ↑ Yusufu, Mayinuer; Bukhari, Javaria; Yu, Xiaobin; Lin, Timothy P.H.; Lam, Dennis S.C.; Wang, Ningli (September–October 2021). "Challenges in Eye Care in the Asia-Pacific Region". Asia-Pacific Journal of Ophthalmology 10 (5): 423–429. doi:10.1097/APO.0000000000000391. PMID 34516436.
- ↑ Du, Y.F.; Liu, H.R.; Zhang, Y.; Bai, W.L.; Li, R.Y.; Sun, R.Z.; Wang, N.L. (18 January 2022). "Prevalence of cataract and cataract surgery in urban and rural Chinese populations over 50 years old: a systematic review and Meta-analysis". Int J Ophthalmol. 15 (1): 141–149. doi:10.18240/ijo.2022.01.21. PMID 35047369.
- ↑ 134.0 134.1 Vs Murthy, G.; Jain, B.; Shamanna, B; Subramanyam, D. (2014). "Improving cataract services in the Indian context". Community Eye Health 27 (85): 4–5. PMID 24966453.
- ↑ 135.0 135.1 Barnagarwala, Tabassum (23 February 2022). "When India's mass eye camps leave people blind.". Scroll.in. https://scroll.in/article/1017951/when-indias-mass-eye-camps-leave-people-blind.
- ↑ Mabiyan, Rashmi (25 October 2017). "Cataract prevalent in India despite largest number of surgeries: Dr Mahipal S Sachdev, Centre for Sight". https://health.economictimes.indiatimes.com/news/hospitals/cataract-prevalent-in-india-despite-largest-number-of-surgeries-dr-mahipal-s-sachdev-centre-for-sight/61220611.
- ↑ Wong, T.Y. (October 2005). "Cataract surgery programmes in Africa". The British Journal of Ophthalmology 89 (10): 1231–1232. doi:10.1136/bjo.2005.072645. PMID 16170103.
- ↑ Aboobaker, S.; Courtright, P. (January–March 2016). "Barriers to Cataract Surgery in Africa: A Systematic Review". Middle East Afr J Ophthalmol 23 (1): 145–9. doi:10.4103/0974-9233.164615. PMID 26957856.
- ↑ The Epidemic of Cataracts in sub-Saharan Africa (Report). https://www.embracerelief.org/wp-content/uploads/2019/06/The-Epidemic-of-Cataracts-in-sub-Saharan-Africa-.pdf. Retrieved 24 February 2023.
- ↑ Lewallen, S.; Courtright, P; Etya'ale, D.; Mathenge, W.; Schmidt, E; Oye, J.; Clark, A; Williams, T. (October 2013). "Cataract incidence in sub-Saharan Africa: what does mathematical modeling tell us about geographic variations and surgical needs?". Ophthalmic Epidemiol. 20 (5): 260–6. doi:10.3109/09286586.2013.823215. PMID 24070099. https://pubmed.ncbi.nlm.nih.gov/24070099/. Retrieved 2023-02-26.
- ↑ "Speedy Eye Surgery for Cataract Patients". Department of Health and Wellness (Western Cape Government). 20 July 2016. https://www.westerncape.gov.za/general-publication/speedy-eye-surgery-cataract-patients.
- ↑ "Pretoria Eye Institute gives 70 patients the gift of sight". http://www.eyeinstitute.co.za/newspages/PTACataract.aspx.
- ↑ 143.0 143.1 143.2 Lecuona, K.; Cook, C. (2011). "South Africa's cataract surgery rates: why are we not meeting our targets?". South African Medical Journal 101 (8): 510–512. PMID 21920119. https://pubmed.ncbi.nlm.nih.gov/21920119/. Retrieved 2023-02-25. Full text available via The Scientific Electronic Library Online (SciELO) SA
- ↑ Lansingh, Van C.; Resnikoff, Serge; Tingley-Kelley, Kimberly; Nano, María E.; Martens, Marion; Silva, Juan C.; Duerksen, Rainald; Carter, Marissa J. (19 March 2010). "Cataract Surgery Rates in Latin America: A Four-Year Longitudinal Study of 19 Countries". Ophthalmic Epidemiology 17 (2): 75–81. doi:10.3109/09286581003624962. PMID 20302429. https://www.tandfonline.com/doi/abs/10.3109/09286581003624962?journalCode=iope20. Retrieved 4 March 2023.
- ↑ Batlle, Juan Francisco; Lansingh, Van Charles; Silva, Juan Carlos; Eckert, Kristen Allison; Resnikoff, Serge (2014). "The Cataract Situation in Latin America: Barriers to Cataract Surgery". American Journal of Ophthalmology 158 (2): 242–250. doi:10.1016/j.ajo.2014.04.019. ISSN 0002-9394. PMID 24792101. https://www.sciencedirect.com/science/article/pii/S0002939414002220. full text available at Researchgate
- ↑ Limburg, H.; Silva, J.C.; Foster, A. (2009). Cataract in Latin America: findings from nine recent surveys. (Report). 25. Rev Panam Salud Publica. pp. 449–55. https://www.scielosp.org/pdf/rpsp/v25n5/11.pdf. Retrieved 2023-03-04.
- ↑ "Social and Economic Impacts of Restoring Sight". The Fred Hollows Foundation. 7 April 2017. https://www.hollows.org/au/research/social-and-economic-impacts-of-restoring-sight.
- ↑ Finger, R.P.; Kupitz, D.G.; Fenwick, E.; Balasubramaniam, B.; Ramani, R.V.; Holz, F.G.; Gilbert, C.E. (August 2012). "The impact of successful cataract surgery on quality of life, household income and social status in South India". PLOS ONE 7 (8): e44268. doi:10.1371/journal.pone.0044268. PMID 22952945. Bibcode: 2012PLoSO...744268F.
- ↑ 149.0 149.1 Foster, Allen, ed (2000). "Vision 2020: the cataract challenge.". Community Eye Health 13 (34): 17–19. PMID 17491949.
- ↑ "Vision 2020". American Academy of Ophthalmology. https://www.aao.org/education/vision-2020.
- ↑ "VISION 2020". International Agency for the Prevention of Blindness. https://www.iapb.org/about/history/vision-2020/.
- ↑ 152.0 152.1 152.2 Abdulhussein, Dalia; Hussein, Mina Abdul (30 September 2022). "WHO Vision 2020: Have We Done It?". Ophthalmic Epidemiology: 1–9. doi:10.1080/09286586.2022.2127784. PMID 36178293. https://www.tandfonline.com/doi/full/10.1080/09286586.2022.2127784. Retrieved 6 March 2023.
- ↑ "Facts About Cataract | National Eye Institute". https://nei.nih.gov/health/cataract/cataract_facts.
- ↑ 154.0 154.1 Struck, M.C. (October 2015). "Long-term Results of Pediatric Cataract Surgery and Primary Intraocular Lens Implantation From 7 to 22 Months of Life.". JAMA Ophthalmol. 133 (10): 1180–1183. doi:10.1001/jamaophthalmol.2015.2062. PMID 26111188.
- ↑ Monahan, Patrick (9 March 2016). "Eyes can regenerate their own lenses after cataract surgery". Science. https://www.science.org/content/article/eyes-can-regenerate-their-own-lenses-after-cataract-surgery. Retrieved 10 March 2023.
- ↑ Lin, Haotian; Ouyang, Hong; Zhu, Jie; Huang, Shan; Liu, Zhenzhen; Chen, Shuyi; Cao, Guiqun; Li, Gen et al. (9 March 2016). "Lens regeneration using endogenous stem cells with gain of visual function". Nature 531 (7594): 323–328. doi:10.1038/nature17181. PMID 26958831. Bibcode: 2016Natur.531..323L.
- ↑ Alió, Jorge L.; Nowrouzi, Ali (29 August 2022). "Immediately sequential bilateral cataract surgery importance during the COVID-19 pandemic.". Saudi J Ophthalmol. 36 (2): 124–128. doi:10.4103/sjopt.sjopt_131_22. PMID 36211314.
- ↑ Obuchowska, I; Mariak, Z. (2006). "Jednoczesna operacja zaćmy w obojgu oczach--zalety i wady [Simultaneous bilateral cataract surgery--advantages and disadvantages]" (in Polish). Klin Oczna 108 (7–9): 353–6. PMID 17290841.
Further reading
- "The clinical effectiveness and cost-effectiveness of second-eye cataract surgery: a systematic review and economic evaluation". Health Technology Assessment (NIHR Journals Library) 18 (68): 1–205, v–vi. November 2014. doi:10.3310/hta18680. 18.68. PMID 25405576.
- "Ch: 11. Cataract Surgery". Essential Surgery. Disease Control Priorities. 1 (3rd ed.). The International Bank for Reconstruction and Development / The World Bank. 2015. doi:10.1596/978-1-4648-0346-8. ISBN 978-1-4648-0346-8. https://www.ncbi.nlm.nih.gov/books/NBK333487/. Retrieved 2017-08-16.
- Tasman, William, ed (2006). "Duane's Opthalmology". Lippincott, Williams and Wilkins. http://www.oculist.net/downaton502/prof/ebook/duanes/index.html.
External links
