Biology:Sf caspase-1
| Sf caspase-1 | |||||||
|---|---|---|---|---|---|---|---|
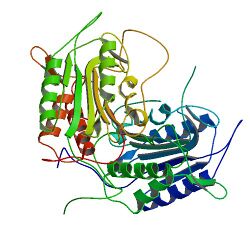 Sf caspase-1 structure by Ni et al. 2006 | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | N/A | ||||||
| PDB | 2NN3 (ECOD) | ||||||
| RefSeq (mRNA) | U81510.1 | ||||||
| RefSeq (Prot) | AAC47442.1 | ||||||
| UniProt | P89116 | ||||||
| |||||||
The protein Sf caspase-1 is the insect ortholog of the human effector caspases CASP3 (CPP32) and CASP7 (MCH3) in the species Spodoptera frugiperda (Fall armyworm).[1][2] It was identified as the target of the baculoviral caspase inhibitor protein P35, which it cleaves and by which it is inhibited.[1] Like other caspases, Sf caspase-1 is an aspartate-specific cysteine protease that is produced as an inactive proenzyme and becomes activated by autocatalytic cleavage.[1] The Sf caspase-1 proenzyme is cleaved after the amino acid residues Asp-28 and Asp-195, resulting in a smaller 12 kDa fragment and a larger 19 kDa fragment.[1][2] Just like with human caspases CASP3 or CASP7, the two cleavage fragments form heterodimers, which again form biologically active dimers-of-heterodimers consisting of two smaller and two larger fragments.[2] Some experiments also showed cleavage of Sf caspase-1 at the residue Asp-184, resulting in an 18 kDa instead of 19 kDa fragment, however this result is likely an in vitro artefact.[2] The insect immunophilin FKBP46 is a substrate of Sf caspase-1, which cleaves full length FKBP46 (~46 kDa) resulting in a ~25 kDa fragment.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP46, is the target of the baculovirus antiapoptotic protein p35". The Journal of Biological Chemistry 272 (3): 1421–4. January 1997. doi:10.1074/jbc.272.3.1421. PMID 8999805.
- ↑ 2.0 2.1 2.2 2.3 "Crystal structure of an invertebrate caspase". The Journal of Biological Chemistry 279 (8): 7001–8. February 2004. doi:10.1074/jbc.M312472200. PMID 14645217.
 |

