Chemistry:Bisoctrizole
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
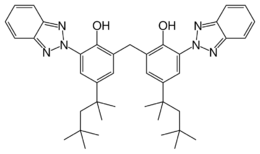
2,2′-Methylenebis[6-(2H-1,2,3-benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol] | |
| Other names
UV-360
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C41H50N6O2 | |
| Molar mass | 658.88 g/mol |
| Melting point | 195.7 °C (384.3 °F; 468.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bisoctrizole (INN[1]/USAN,[2] marketed by BASF as Tinosorb M, by DSM Nutritional Products as Parsol Max , by Everlight Chemical as Eversorb M, and by MPI as Milestab 360, INCI methylene bis-benzotriazolyl tetramethylbutylphenol) is a phenolic benzotriazole that is added to sunscreens to absorb UV rays.[3] It is a broad-spectrum ultraviolet radiation absorber, absorbing UVB as well as UVA rays.[3] It also reflects and scatters UV.[citation needed]
Bisoctrizole is what is termed is a hybrid UV absorber, which has been described as an organic UV filter produced in microfine organic particles (< 200 nm),[verification needed][4][better source needed][5][non-primary source needed] like microfine zinc oxide and titanium dioxide.[dubious ][citation needed] Where other organic UV absorbers dissolved in either the oily or aqueous phases, bisoctrizole dissolves poorly in both.[citation needed]
Hence, bisoctrizole is formulated in sunscreen preparations as a 50% suspension, the absorber added to the water phase,[contradictory] and mineral micropigments usually added to the oil phase.[citation needed] The bisoctrizole particles are stabilized by the surfactant decyl glucoside.[citation needed] The compound shows very little photodegradation, and has a stabilizing effect on other UV absorbers, octyl methoxycinnamate (octinoxate) in particular.[citation needed]
In primary research reports, bisoctrizole has been reported to minimally penetrate skin,[6][non-primary source needed] and has been described as lacking estrogenic effects in vitro.[7][non-primary source needed]
References
- ↑ WHO Staff (2005). "Recommended International Nonproprietary Names: List 54, International Nonproprietary Names for Pharmaceutical Substances (INN) [Entry 'bisoctrizolum'"]. WHO Drug Information 19 (3). http://apps.who.int/medicinedocs/en/d/Js7918e/8.html. Retrieved July 5, 2022.
- ↑ National Library of Medicine Staff (July 5, 2022). "Bisoctrizole". https://chem.nlm.nih.gov/chemidplus/rn/103597-45-1.
- ↑ 3.0 3.1 Latha, MS; Martis, Jacintha; Shobha, V; Shinde, Rutuja Sham; Bangera, Sudhakar; Krishnankutty, Binny; Bellary, Shantala; Varughese, Sunoj et al. (January 2013). "Sunscreening Agents A Review". Journal of Clinical and Aesthetic Dermatology 6 (1): 16–26. PMID 23320122.
- ↑ Ciba Staff (July 5, 2022). "TINOSORB® M, Broad-spectrum UV Filter for the Water Phase". https://www.carecreations.basf.com/products-formulation/products/products-detail/TINOSORB%20M/30482916.
- ↑ Herzog, B.; Mongiat, S.; Deshayes, C.; Neuhaus, M.; Sommer, K.; Mantler, A. (2002). "In vivo and in vitro assessment of UVA protection by sunscreen formulations containing either butyl methoxy dibenzoyl methane, methylene bis-benzotriazolyl tetramethylbutylphenol, or microfine ZnO". International Journal of Cosmetic Science 24 (3): 170–85. doi:10.1046/j.1467-2494.2002.00137.x. PMID 18498509.[non-primary source needed]
- ↑ "In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen". Skin Pharmacol Physiol 20 (1): 10–20. 2007. doi:10.1159/000096167. PMID 17035717.[non-primary source needed]
- ↑ "Lack of binding to isolated estrogen or androgen receptors, and inactivity in the immature rat uterotrophic assay, of the ultraviolet sunscreen filters Tinosorb M-active and Tinosorb S". Regul Toxicol Pharmacol 34 (3): 287–91. December 2001. doi:10.1006/rtph.2001.1511. PMID 11754532.[non-primary source needed]
External links
- Ciba® TINOSORB® M – Microfine UV absorber with Triple Action
- http://www.dermatologytimes.com/dermatologytimes/article/articleDetail.jsp?id=159652
- NEW-WAVE SUNSCREENS – Active ingredient makers are frustrated by the long list of sunscreens and UV-A testing protocols that are still awaiting FDA decisions, C&EN Cover Story
- https://www.fda.gov/ohrms/dockets/dockets/05n0446/05n-0446-bkg0001-03-Tab-01-vol2.pdf
 |

