Chemistry:Phase separation
From HandWiki
Revision as of 19:31, 28 June 2021 by imported>Corlink (simplify)
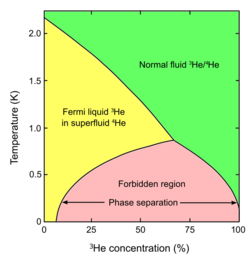
A phase diagram for two isotopes of helium, showing at bottom a range of temperatures and ratios at which they will phase-separate.
Phase separation is the creation of two distinct phases from a single homogeneous mixture.[1] The most common type of phase separation is between two immiscible liquids such as oil and water. Colloids are formed by phase separation, though not all phase separation forms colloids - for example oil and water can form separated layers under gravity rather than remaining as microscopic droplets in suspension.
See also
References
- ↑ "Phase separation". IUPAC Compendium of Chemical Terminology (the "Gold Book") (2nd ed.). Oxford: Blackwell Scientific Publications. 1997. doi:10.1351/goldbook.P04534. ISBN 0-9678550-9-8. https://goldbook.iupac.org/html/P/P04534.html.
Further reading
- Khabibullaev, Pulat K.; Saidov, Abdulla (April 2013). Phase Separation in Soft Matter Physics: Micellar Solutions, Microemulsions, Critical Phenomena. Berlin Heidelberg: Springer. ISBN 978-3-662-09278-1.
 |