Physics:Coulomb constant
| Value of k | Units |
|---|---|
| 8.9875517923(14)×109 | N·m2/C2 |
| 14.3996 | eV·Å·e−2 |
| 10−7 | (N·s2/C2)c2 |
The Coulomb constant, the electric force constant, or the electrostatic constant (denoted ke, k or K) is a proportionality constant in electrostatics equations. In SI base units it is equal to 8.9875517923(14)×109 kg⋅m3⋅s−4⋅A−2.[1] It was named after the French physicist Charles-Augustin de Coulomb (1736–1806) who introduced Coulomb's law.[2][3]
Value of the constant
The Coulomb constant is the constant of proportionality in Coulomb's law,
- [math]\displaystyle{ \mathbf{F} = k_\text{e}\frac{Qq}{r^2}\mathbf{\hat{e}}_r }[/math]
where êr is a unit vector in the r-direction.[4] In SI:
- [math]\displaystyle{ k_\text{e} = \frac{1}{4\pi\varepsilon_0}, }[/math]
where [math]\displaystyle{ \varepsilon_0 }[/math] is the vacuum permittivity. This formula can be derived from Gauss' law,
-
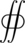 [math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = \frac{Q}{\varepsilon_0} }[/math]
[math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = \frac{Q}{\varepsilon_0} }[/math]
Taking this integral for a sphere, radius r, centered on a point charge, the electric field points radially outwards and is normal to a differential surface element on the sphere with constant magnitude for all points on the sphere.
-
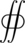 [math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = |\mathbf{E}|\int_{S} dA = |\mathbf{E}| \times 4\pi r^{2} }[/math]
[math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = |\mathbf{E}|\int_{S} dA = |\mathbf{E}| \times 4\pi r^{2} }[/math]
Noting that E = F/q for some test charge q,
- [math]\displaystyle{ \begin{align} \mathbf{F} &= \frac{1}{4\pi\varepsilon_0}\frac{Qq}{r^2}\mathbf{\hat{e}}_r = k_\text{e}\frac{Qq}{r^2}\mathbf{\hat{e}}_r \\[8pt] \therefore k_\text{e} &= \frac{1}{4\pi\varepsilon_0} \end{align} }[/math]
Coulomb's law is an inverse-square law, and thereby similar to many other scientific laws ranging from gravitational pull to light attenuation. This law states that a specified physical quantity is inversely proportional to the square of the distance.[math]\displaystyle{ \text{intensity} = \frac{1}{d^2} }[/math]In some modern systems of units, the Coulomb constant ke has an exact numeric value; in Gaussian units ke = 1, in Heaviside–Lorentz units (also called rationalized) ke = 1/4π. This was previously true in SI when the vacuum permeability was defined as μ0 = 4π×10−7 H⋅m−1. Together with the speed of light in vacuum c, defined as 299792458 m/s, the vacuum permittivity ε0 can be written as 1/μ0c2, which gave an exact value of[5]
- [math]\displaystyle{ \begin{align} k_\text{e} = \frac{1}{4\pi\varepsilon_0}=\frac{c^2\mu_0}{4\pi}&=c^2\times (10^{-7}\ \mathrm{H{\cdot}m}^{-1})\\ &= 8.987\ 551\ 787\ 368\ 1764\times 10^9~\mathrm{N{\cdot}m^2{\cdot}C^{-2}}. \end{align} }[/math]
Since the redefinition of SI base units,[6][7] the Coulomb constant is no longer exactly defined and is subject to the measurement error in the fine structure constant, as calculated from CODATA 2018 recommended values being[1]
- [math]\displaystyle{ k_\text{e} = 8.987\,551\,7923\,(14)\times 10^9\;\mathrm{kg{\cdot}m^{3}{\cdot}s^{-4}{\cdot}A^{-2}} . }[/math]
Use
The Coulomb constant is used in many electric equations, although it is sometimes expressed as the following product of the vacuum permittivity constant:
- [math]\displaystyle{ k_\text{e} = \frac{1}{4 \pi \varepsilon_0}. }[/math]
The Coulomb constant appears in many expressions including the following:
- Coulomb's law
- [math]\displaystyle{ \mathbf{F}=k_\text{e}{Qq\over r^2}\mathbf{\hat{e}}_r. }[/math]
- Electric potential energy
- [math]\displaystyle{ U_\text{E}(r) = k_\text{e}\frac{Qq}{r}. }[/math]
- Electric field
- [math]\displaystyle{ \mathbf{E} = k_\text{e} \sum_{i=1}^N \frac{Q_i}{r_i^2} \mathbf{\hat{r}}_i. }[/math]
See also
References
- ↑ 1.0 1.1 Derived from ke = 1/(4πε0) – "2018 CODATA Value: vacuum electric permittivity". The NIST Reference on Constants, Units, and Uncertainty. NIST. 20 May 2019. http://physics.nist.gov/cgi-bin/cuu/Value?ep0.
- ↑ Britannica, The Editors of Encyclopaedia. "Coulomb". https://www.britannica.com/science/coulomb.
- ↑ Britannica, The Editors of Encyclopaedia. "Charles-Augustin de Coulomb". https://www.britannica.com/biography/Charles-Augustin-de-Coulomb.
- ↑ Tomilin, K. (1999). "Fine-structure constant and dimension analysis". European Journal of Physics 20 (5): L39–L40. doi:10.1088/0143-0807/20/5/404. Bibcode: 1999EJPh...20L..39T.
- ↑ Coulomb's constant, HyperPhysics
- ↑ BIPM statement: Information for users about the proposed revision of the SI, https://www.bipm.org/utils/common/pdf/SI-statement.pdf
- ↑ "Decision CIPM/105-13 (October 2016)". http://www.bipm.org/en/committees/cipm/meeting/105.html. The day is the 144th anniversary of the Metre Convention.

