Chemistry:Dimethylaluminium chloride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H12Al2Cl2 | |
| Molar mass | 185.00 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.996 g cm−3 |
| Melting point | −21 °C (−6 °F; 252 K) |
| Boiling point | 126–127 °C (259–261 °F; 399–400 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H250, H260, H314 | |
| P210, P222, P223, P231, P231+232, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P302+335+334Script error: No such module "Preview warning".Category:GHS errors, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P303+361+353, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P321, P363, P370+378, P402+404, P403+235 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethylaluminium chloride is an organoaluminium compound with the chemical formula [(CH3)2AlCl]2. It behaves similarly to diethylaluminium chloride but is more expensive. Hence, it is less commonly used.[2]
Like other organoaluminium chlorides, dimethylaluminium chloride is a Lewis acid. This property is exploited by the use of dimethylaluminium chloride to induce some Diels-Alder reactions.[3]
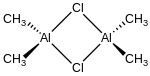
Structure and bonding
Compounds of the empirical formula AlR2Cl (R = alkyl, aryl) usually exist as dimers with the formula (R2Al)2(μ-Cl)2. The bridging ligands, indicated by the prefix "μ-", are halides, not the organic substituents. The aluminium adopts a tetrahedral geometry and follows the octet rule.[4][5] By contrast, triethylaluminium and trimethylaluminium feature bridging alkyl groups and these compounds violate the octet rule.
Safety
Dimethylaluminium chloride is not only flammable but pyrophoric.
References
- ↑ "Aluminum, chlorodimethyl-" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/79147#section=Safety-and-Hazards.
- ↑ Snider, Barry B. (2001). "Dimethylaluminum Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd297. ISBN 0-471-93623-5.
- ↑ Danheiser, Rick L.; Renslo, Adam R.; Amos, David T.; Wright, Graham T. (2003). "Preparation of Substituted Pyridines Via Regiocontrolled [4 + 2] Cycloadditions of Oximinosulfonates: Methyl 5-Methylpyridine-2-Carboxylate". Organic Syntheses 80: 133. doi:10.15227/orgsyn.080.0133.
- ↑ Brendhaugen, Kristen; Haaland, Arne; Novak, David P.; Østvold, Terje; Bjørseth, Alf; Powell, D. L. (1974). "The Molecular Structure of Dimethylaluminium Chloride Dimer, [(CH3)2AlCl]2 Redetermined by Gas Phase Electron Diffraction". Acta Chemica Scandinavica 28a: 45–47. doi:10.3891/acta.chem.scand.28a-0045.
- ↑ McMahon, C. Niamh; Francis, Julie A.; Barron, Andrew R. (1997). "Molecular Atructure of [(tBu)2Al(μ-Cl)]2". Journal of Chemical Crystallography 27 (3): 191–194. doi:10.1007/BF02575988.
 |

