Chemistry:Vargulin
| |
| Names | |
|---|---|
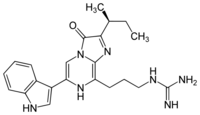
| IUPAC name
2-[3-[2-[(2S)-butan-2-yl]-6-(1H-indol-3-yl)-3-oxo-7H-imidazo[2,
1-c]pyrazin-8-yl]propyl]guanidine
| |
| Other names
Cypridina luciferin, cypridinid luciferin, Vargula luciferin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C22H27N7O | |
| Molar mass | 405.506 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vargulin,[1] also called Cypridinid luciferin,[2] Cypridina luciferin, or Vargula luciferin, is the luciferin found in the ostracod Cypridina hilgendorfii, also named Vargula hilgendorfii.[3] These bottom dwelling ostracods emit a light stream into water when disturbed presumably to deter predation. Vargulin is also used by the midshipman fish, Porichthys.
History
A partial extraction procedure was developed in 1935 which involved reacting the compound with benzoyl chloride to allow it to be separated from the water-soluble components.[4] The compound was first isolated and purified to crystals by Osamu Shimomura.[5] The structure of the compound was confirmed some years later.[6] Feeding experiments suggest that the compound is synthesized in the animal from three amino-acids: tryptophan, isoleucine, and arginine.[7]
Biochemistry
Vargulin is oxidized by the Vargula luciferase,[8] a 62 kDa enzyme, to produce blue light at 462 nm (max emission, detected with a 425 to 525 nm filter). The vargulin does not cross react with luciferases using coelenterazine or Firefly luciferin.
Uses
Vargulin (with the associated luciferase) has applications in biotechnology:
- in a variety of assays, to report gene or gene expression after luciferase have been genetically introduced in cells,
- to detect ATP, that is used in the vargulin/luciferase reaction (cell viability assays).[9]
Although less stable, the Cypridina system is useful because can be used in multiplex assays with other (red-emitting) luciferin assays.
References
- ↑ Campbell, A. K.; Herring, P. J. (1990). "Imidazolopyrazine bioluminescence in copepods and other marine organisms". Marine Biology (Springer Science and Business Media LLC) 104 (2): 219–225. doi:10.1007/bf01313261. ISSN 0025-3162.
- ↑ Morin, James G. (2011). "Based on a review of the data, use of the term 'cypridinid' solves the Cypridina/Vargula dilemma for naming the constituents of the luminescent system of ostracods in the family Cypridinidae". Luminescence (Wiley) 26 (1): 1–4. doi:10.1002/bio.1282. ISSN 1522-7235. PMID 19862683.
- ↑ Shimomura, O. (2006). Bioluminescence: Chemical Principles and Methods. World Scientific Publishing. ISBN 978-981-256-801-4.
- ↑ Anderson, RS (1935). "Studies on Bioluminescence : II. the Partial Purification of Cypridina Luciferin.". The Journal of General Physiology 19 (2): 301–5. doi:10.1085/jgp.19.2.301. PMID 19872927.
- ↑ Shimomura, O; Goto, T; Hirata, Y (1957). "Crystalline Cypridina Luciferin". Bulletin of the Chemical Society of Japan 30 (8): 929–933. doi:10.1246/bcsj.30.929. http://naosite.lb.nagasaki-u.ac.jp/dspace/bitstream/10069/20882/1/BullCSJ30_929.pdf.
- ↑ Kishi Y, Goto; T, Hirata Y; Shiromura O; Johnson FH (1966). "Cypridina bioluminescence. I. Structure of Cypridina luciferin". Tetrahedron Lett. 7 (29): 3427–3436. doi:10.1016/S0040-4039(01)82806-9.
- ↑ Oba, Y; Kato, S; Ojika, M; Inouye, S (2002). "Biosynthesis of luciferin in the sea firefly, Cypridina hilgendorfii: l-tryptophan is a component in Cypridina luciferin". Tetrahedron Letters 43 (12): 2389–2392. doi:10.1016/S0040-4039(02)00257-5.
- ↑ "Cloning and expression of cDNA for the luciferase from the marine ostracod Vargula hilgendorfii". Proceedings of the National Academy of Sciences 86 (17): 6567–71. 1989. doi:10.1073/pnas.86.17.6567. PMID 2771943. Bibcode: 1989PNAS...86.6567T.
- ↑ "Luciferase Reporters". https://www.thermofisher.com/vn/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/luciferase-reporters.html.
External links
 |

