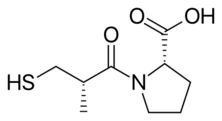
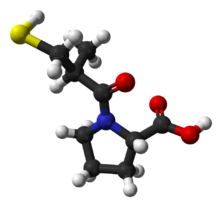
Chemistry:Captopril
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈkæptəprɪl/ |
| Trade names | Capoten, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682823 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70–75% |
| Metabolism | Liver |
| Elimination half-life | 1.9 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C9H15NO3S |
| Molar mass | 217.28 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Captopril, sold under the brand name Capoten among others, is an angiotensin-converting enzyme (ACE) inhibitor used for the treatment of hypertension and some types of congestive heart failure. Captopril was the first oral ACE inhibitor found for the treatment of hypertension.[2] It does not cause fatigue as associated with beta-blockers. Due to the adverse drug event of causing hyperkalemia, as seen with most ACE Inhibitors, the medication is usually paired with a diuretic.
Captopril was patented in 1976 and approved for medical use in 1980.[3]
Structure–activity relationship
Captopril has an L-proline group which allows for it to be more bioavailable within oral formulations. The thiol moiety within the molecule has been associated with two significance adverse effects: the hapten or immune response. This immune response, also known as agranulocytosis, can explain the adverse drug events which may be seen in captopril with the allergic response, which would be: hives, severe stomach pain, difficulty breathing, swelling of the face, lips, tongue or throat.[4]
In terms of interaction with the enzyme, the molecule's thiol moiety will attach to the binding site of the ACE enzyme. This will inhibit the port at which the angiotensin-1 molecule would normally bind, therefore inhibiting the downstream effects within the renin-angiotensin system.
Medical uses
Captopril's main uses are based on its vasodilation and inhibition of some renal function activities. These benefits are most clearly seen in:
- Hypertension
- Cardiac conditions such as congestive heart failure and after myocardial infarction
- Preservation of kidney function in diabetic nephropathy.
Additionally, it has shown mood-elevating properties in some patients. This is consistent with the observation that animal screening models indicate putative antidepressant activity for this compound, although one study has been negative. Formal clinical trials in depressed patients have not been reported.[6]
It has also been investigated for use in the treatment of cancer.[7] Captopril stereoisomers were also reported to inhibit some metallo-β-lactamases.[8]
Adverse effects
Adverse effects of captopril include cough due to increase in the plasma levels of bradykinin, angioedema, agranulocytosis, proteinuria, hyperkalemia, taste alteration, teratogenicity, postural hypotension, acute renal failure, and leukopenia.[9] Except for postural hypotension, which occurs due to the short and fast mode of action of captopril, most of the side effects mentioned are common for all ACE inhibitors. Among these, cough is the most common adverse effect. Hyperkalemia can occur, especially if used with other drugs which elevate potassium level in blood, such as potassium-sparing diuretics. Other side effects are:
- Itching
- Headache
- Tachycardia
- Chest pain
- Palpitations
- Dysgeusia
- Weakness
The adverse drug reaction (ADR) profile of captopril is similar to other ACE inhibitors, with cough being the most common ADR.[10] However, captopril is also commonly associated with rash and taste disturbances (metallic or loss of taste), which are attributed to the unique thiol moiety.[11]
Overdose
ACE inhibitor overdose can be treated with naloxone.[12][13][14]
History
In the late 1960s, John Vane of the Royal College of Surgeons of England was working on mechanisms by which the body regulates blood pressure.[15] He was joined by Sérgio Henrique Ferreira of Brazil, who had been studying the venom of a Brazilian pit viper, the jararaca (Bothrops jararaca), and brought a sample of the viper's venom. Vane's team found that one of the venom's peptides selectively inhibited the action of angiotensin-converting enzyme (ACE), which was thought to function in blood pressure regulation; the snake venom functions by severely depressing blood pressure. During the 1970s, ACE was found to elevate blood pressure by controlling the release of water and salts from the kidneys.
Captopril, an analog of the snake venom's ACE-inhibiting peptide, was first synthesized in 1975 by three researchers at the U.S. drug company E.R. Squibb & Sons Pharmaceuticals (now Bristol-Myers Squibb): Miguel Ondetti, Bernard Rubin, and David Cushman. Squibb filed for U.S. patent protection on the drug in February 1976, which was granted in September 1977, and captopril was approved for medical use in 1980.[3] It was the first ACE inhibitor developed and was considered a breakthrough both because of its mechanism of action and also because of the development process.[16][17] In the 1980s, Vane received the Nobel prize and was knighted for his work and Ferreira received the National Order of Scientific Merit from Brazil.
The development of captopril was among the earliest successes of the revolutionary concept of ligand-based drug design. The renin–angiotensin–aldosterone system had been extensively studied in the mid-20th century, and this system presented several opportune targets in the development of novel treatments for hypertension. The first two targets that were attempted were renin and ACE. Captopril was the culmination of efforts by Squibb's laboratories to develop an ACE inhibitor.
Ondetti, Cushman, and colleagues built on work that had been done in the 1960s by a team of researchers led by John Vane at the Royal College of Surgeons of England. The first breakthrough was made by Kevin K.F. Ng[18][19][20] in 1967, when he found the conversion of angiotensin I to angiotensin II took place in the pulmonary circulation instead of in the plasma. In contrast, Sergio Ferreira[21] found bradykinin disappeared in its passage through the pulmonary circulation. The conversion of angiotensin I to angiotensin II and the inactivation of bradykinin were thought to be mediated by the same enzyme.
In 1970, using bradykinin potentiating factor (BPF) provided by Sergio Ferreira,[22] Ng and Vane found the conversion of angiotensin I to angiotensin II was inhibited during its passage through the pulmonary circulation. BPF was later found to be a peptide in the venom of a lancehead viper (Bothrops jararaca), which was a “collected-product inhibitor” of the converting enzyme. Captopril was developed from this peptide after it was found via QSAR-based modification that the terminal sulfhydryl moiety of the peptide provided a high potency of ACE inhibition.[23]
Captopril gained FDA approval on April 6, 1981. The drug became a generic medicine in the U.S. in February 1996, when the market exclusivity held by Bristol-Myers Squibb for captopril expired.
Chemical synthesis
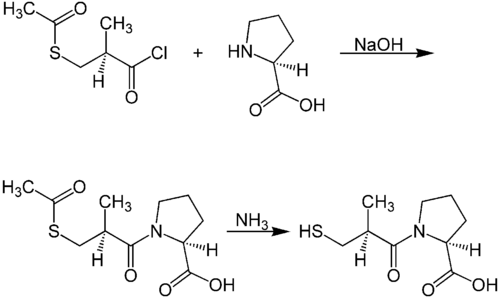
A chemical synthesis of captopril by treatment of L-proline with (2S)-3-acetylthio-2-methylpropanoyl chloride under basic conditions (NaOH), followed by aminolysis of the protective acetyl group to unmask the drug's free thiol, is depicted in the figure at right.[24]
| Captopril synthesis 1 | Captopril synthesis 2 |
|---|---|
| center|500px|Patents:[25][26][27] Design and synthesis:[28][29] Improved synthesis:[30]]] |
Procedure 2 taken out of patent US4105776. See examples 28, 29a and 36.
Mechanism of action
Captopril blocks the conversion of angiotensin I to angiotensin II and prevents the degradation of vasodilatory prostaglandins, thereby inhibiting vasoconstriction and promoting systemic vasodilation.[31]
Pharmacokinetics
Unlike the majority of ACE inhibitors, captopril is not administered as a prodrug (the only other being lisinopril).[32] About 70% of orally administered captopril is absorbed. Bioavailability is reduced by presence of food in stomach. It is partly metabolised and partly excreted unchanged in urine.[33] Captopril also has a relatively poor pharmacokinetic profile. The short half-life necessitates dosing two or three times per day, which may reduce patient compliance. Captopril has a short half-life of 2–3 hours and a duration of action of 12–24 hours.
See also
References
- ↑ "List of nationally authorised medicinal products Active substance: captopril" (in en). European Medicines Agency. 26 November 2020. https://www.ema.europa.eu/en/documents/psusa/captopril-list-nationally-authorised-medicinal-products-psusa/00000535/202004_en.pdf.
- ↑ "Medical intelligence drug therapy: captopril" (in EN). The New England Journal of Medicine 306 (4): 214–219. January 1982. doi:10.1056/nejm198201283060405. PMID 7033784.
- ↑ 3.0 3.1 (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 467. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA467.
- ↑ "Captopril: Uses, Dosage, Side Effects" (in en). https://www.drugs.com/captopril.html.
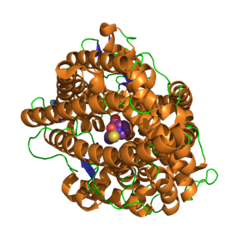
- ↑ "High-resolution crystal structures of Drosophila melanogaster angiotensin-converting enzyme in complex with novel inhibitors and antihypertensive drugs". Journal of Molecular Biology 400 (3): 502–517. July 2010. doi:10.1016/j.jmb.2010.05.024. PMID 20488190.
- ↑ "Novel Pharmacological Approaches to the Treatment of Depression". Psychopharmacology (The Fourth Generation of Progress ed.). Raven Press. 1995. pp. 1143–1153. ISBN 978-0-7817-0166-2. https://acnp.org/g4/GN401000109/Default.htm.
- ↑ "Captopril as a potential inhibitor of lung tumor growth and metastasis". Annals of the New York Academy of Sciences 1138 (1): 65–72. September 2008. doi:10.1196/annals.1414.011. PMID 18837885. Bibcode: 2008NYASA1138...65A.
- ↑ "Structural Basis of Metallo-β-Lactamase Inhibition by Captopril Stereoisomers". Antimicrobial Agents and Chemotherapy 60 (1): 142–150. January 2016. doi:10.1128/AAC.01335-15. PMID 26482303.
- ↑ "Captopril (ACE inhibitor): side effects". lifehugger. 2008-07-09. http://mc.lifehugger.com/moc/157/captopril-ace-inhibitor-side-effects.
- ↑ Australian Medicines Handbook. Adelaide: Australian Medicines Handbook. 2006.
- ↑ "Captopril in the treatment of clinical hypertension and cardiac failure". Lancet 2 (8147): 836–839. October 1979. doi:10.1016/S0140-6736(79)92186-X. PMID 90928.
- ↑ Goldfrank's toxicologic emergencies. New York: McGraw-Hill Education. 2019. p. 953. ISBN 978-1-259-85961-8.
- ↑ Meyler's Side Effects of Analgesics and Anti-inflammatory Drugs, Jeffrey K. Aronson, page 120.
- ↑ "Effect of naloxone on the actions of captopril". Clinical Pharmacology and Therapeutics 38 (5): 560–565. November 1985. doi:10.1038/clpt.1985.224. PMID 2996820.
- ↑ "Drugs with bite: The healing powers of venoms" (in en-US). https://www.newscientist.com/article/dn21775-drugs-with-bite-the-healing-powers-of-venoms/.
- ↑ "From snake venom to ACE inhibitor the discovery and rise of captopril". Pharmaceutical Journal. 2009. http://www.pharmaceutical-journal.com/news-and-analysis/news/from-snake-venom-to-ace-inhibitor-the-discovery-and-rise-of-captopril/10884359.article. Retrieved 2015-01-08.
- ↑ "Chronicals of Drug Discovery, vol. 2.". Journal of Pharmaceutical Sciences 74 (9): 1029–1030. September 1985. doi:10.1002/jps.2600740942.
- ↑ "Conversion of angiotensin I to angiotensin II". Nature 216 (5117): 762–766. November 1967. doi:10.1038/216762a0. PMID 4294626. Bibcode: 1967Natur.216..762N.
- ↑ "Fate of angiotensin I in the circulation". Nature 218 (5137): 144–150. April 1968. doi:10.1038/218144a0. PMID 4296306. Bibcode: 1968Natur.218..144N.
- ↑ "Some properties of angiotensin converting enzyme in the lung in vivo". Nature 225 (5238): 1142–1144. March 1970. doi:10.1038/2251142b0. PMID 4313869. Bibcode: 1970Natur.225.1142N.
- ↑ "The disappearance of bradykinin and eledoisin in the circulation and vascular beds of the cat". British Journal of Pharmacology and Chemotherapy 30 (2): 417–424. June 1967. doi:10.1111/j.1476-5381.1967.tb02148.x. PMID 6036419.
- ↑ "The discovery of captopril". FASEB Journal 17 (8): 788–789. May 2003. doi:10.1096/fj.03-0093life. PMID 12724335.
- ↑ "From viper's venom to drug design: treating hypertension". FASEB Journal 18 (3): 421. March 2004. doi:10.1096/fj.03-1398bkt. PMID 15003987.
- ↑ "Synthesis of captopril starting from an optically active .BETA.-hydroxy acid". Chem. Pharm. Bull. 30 (9): 3139–3146. 1982. doi:10.1248/cpb.30.3139.
- ↑ {{Cite patent|country=DE|number=2703828|title=Prolinderivate und verwandte Verbindungen, Verfahren zu ihrer Herstellung und ihre Verwendung als Arzneimittel [Proline derivatives and related compounds, methods for their manufacturing and their use as a medicinal product]|pubdate=1977-08-18|assign=[[E. R. Squibb#Squibb Company|E.R. Squibb & Sons Inc.| inventor= Cushman DW, Ondetti MA }}
- ↑ Ondetti MA, Cushman DW, US patent 4046889, issued 1977, assigned to Squibb
- ↑ Ondetti MA, Cushman DW, US patent 4105776, issued 1978, assigned to Squibb
- ↑ "Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents". Science 196 (4288): 441–444. April 1977. doi:10.1126/science.191908. PMID 191908. Bibcode: 1977Sci...196..441O.
- ↑ "Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids". Biochemistry 16 (25): 5484–5491. December 1977. doi:10.1021/bi00644a014. PMID 200262.
- ↑ "An improved synthesis of captopril". Journal of Pharmaceutical Sciences 73 (12): 1843–1844. December 1984. doi:10.1002/jps.2600731251. PMID 6396401.
- ↑ Davis's drug guide for nurses (Fourteenth ed.). Philadelphia. 2014-06-05. ISBN 978-0-8036-4085-6. OCLC 881473728.
- ↑ "Angiotensin-converting enzyme inhibitors". Circulation 97 (14): 1411–1420. April 1998. doi:10.1161/01.cir.97.14.1411. PMID 9577953.
- ↑ "Pharmacokinetics of captopril in healthy subjects and in patients with cardiovascular diseases". Clinical Pharmacokinetics 14 (4): 241–259. April 1988. doi:10.2165/00003088-198814040-00002. PMID 3292102.
External links
- "Captopril". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/captopril.
- U.S. Patent 4,046,889
- The story of the discovery of Captopril drugdesign.org
 |