Chemistry:Chromium compounds
Chromium compounds are compounds containing the element chromium (Cr). Chromium is a member of group 6 of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.[3][4]
Common oxidation states
| Oxidation states[note 1][4] | |
|---|---|
| −4 (d10) | Na4[Cr(CO)4][5] |
| −2 (d8) | Na2[Cr(CO)5] |
| −1 (d7) | Na2[Cr2(CO)10] |
| 0 (d6) | Cr(C6H6)2 |
| +1 (d5) | K3[Cr(CN)5NO] |
| +2 (d4) | CrCl2 |
| +3 (d3) | CrCl3 |
| +4 (d2) | K2CrF6 |
| +5 (d1) | K3Cr(O2)4 |
| +6 (d0) | K2CrO4 |
Chromium(0)
Many Cr(0) complexes are known. Bis(benzene)chromium and chromium hexacarbonyl are highlights in organochromium chemistry.
Chromium(II)

Chromium(II) compounds are uncommon, in part because they readily oxidize to chromium(III) derivatives in air. Water-stable chromium(II) chloride CrCl2 that can be made by reducing chromium(III) chloride with zinc. The resulting bright blue solution created from dissolving chromium(II) chloride is stable at neutral pH.[6] Some other notable chromium(II) compounds include chromium(II) oxide CrO, and chromium(II) sulfate CrSO4. Many chromium(II) carboxylates are known. The red chromium(II) acetate (Cr2(O2CCH3)4) is somewhat famous. It features a Cr-Cr quadruple bond.[7]
Chromium(III)
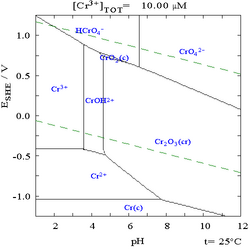
A large number of chromium(III) compounds are known, such as chromium(III) nitrate, chromium(III) acetate, and chromium(III) oxide.[8] Chromium(III) can be obtained by dissolving elemental chromium in acids like hydrochloric acid or sulfuric acid, but it can also be formed through the reduction of chromium(VI) by cytochrome c7.[9] The Cr3+ ion has a similar radius (63 pm) to Al3+ (radius 50 pm), and they can replace each other in some compounds, such as in chrome alum and alum.
Chromium(III) tends to form octahedral complexes. Commercially available chromium(III) chloride hydrate is the dark green complex [CrCl2(H2O)4]Cl. Closely related compounds are the pale green [CrCl(H2O)5]Cl2 and violet [Cr(H2O)6]Cl3. If anhydrous violet[10] chromium(III) chloride is dissolved in water, the violet solution turns green after some time as the chloride in the inner coordination sphere is replaced by water. This kind of reaction is also observed with solutions of chrome alum and other water-soluble chromium(III) salts. A tetrahedral coordination of chromium(III) has been reported for the Cr-centered Keggin anion [α-CrW12O40]5–.[11]
Chromium(III) hydroxide (Cr(OH)3) is amphoteric, dissolving in acidic solutions to form [Cr(H2O)6]3+, and in basic solutions to form [Cr(OH)6]3−. It is dehydrated by heating to form the green chromium(III) oxide (Cr2O3), a stable oxide with a crystal structure identical to that of corundum.[6]
Chromium(VI)
Chromium(VI) compounds are oxidants at low or neutral pH. Chromate anions (CrO2−4) and dichromate (Cr2O72−) anions are the principal ions at this oxidation state. They exist at an equilibrium, determined by pH:
- 2 [CrO4]2− + 2 H+ ⇌ [Cr2O7]2− + H2O
Chromium(VI) oxyhalides are known also and include chromyl fluoride (CrO2F2) and chromyl chloride (CrO2Cl2).[6] However, despite several erroneous claims, chromium hexafluoride (as well as all higher hexahalides) remains unknown, as of 2020.[12]
Sodium chromate is produced industrially by the oxidative roasting of chromite ore with sodium carbonate. The change in equilibrium is visible by a change from yellow (chromate) to orange (dichromate), such as when an acid is added to a neutral solution of potassium chromate. At yet lower pH values, further condensation to more complex oxyanions of chromium is possible.
Both the chromate and dichromate anions are strong oxidizing reagents at low pH:[6]
- Cr2O2−7 + 14 H3O+ + 6 e− → 2 Cr3+ + 21 H2O (ε0 = 1.33 V)
They are, however, only moderately oxidizing at high pH:[6]
- CrO2−4 + 4 H2O + 3 e− → Cr(OH)3 + 5 OH− (ε0 = −0.13 V)
Chromium(VI) compounds in solution can be detected by adding an acidic hydrogen peroxide solution. The unstable dark blue chromium(VI) peroxide (CrO5) is formed, which can be stabilized as an ether adduct CrO5·OR2.[6]
Chromic acid has the hypothetical formula H2CrO4. It is a vaguely described chemical, despite many well-defined chromates and dichromates being known. The dark red chromium(VI) oxide CrO3, the acid anhydride of chromic acid, is sold industrially as "chromic acid".[6] It can be produced by mixing sulfuric acid with dichromate and is a strong oxidizing agent.
Other oxidation states
Compounds of chromium(V) are rather rare; the oxidation state +5 is only realized in few compounds but are intermediates in many reactions involving oxidations by chromate. The only binary compound is the volatile chromium(V) fluoride (CrF5). This red solid has a melting point of 30 °C and a boiling point of 117 °C. It can be prepared by treating chromium metal with fluorine at 400 °C and 200 bar pressure. The peroxochromate(V) is another example of the +5 oxidation state. Potassium peroxochromate (K3[Cr(O2)4]) is made by reacting potassium chromate with hydrogen peroxide at low temperatures. This red brown compound is stable at room temperature but decomposes spontaneously at 150–170 °C.[13]
Compounds of chromium(IV) are slightly more common than those of chromium(V). The tetrahalides, CrF4, CrCl4, and CrBr4, can be produced by treating the trihalides (CrX3) with the corresponding halogen at elevated temperatures. Such compounds are susceptible to disproportionation reactions and are not stable in water. Organic compounds containing Cr(IV) state such as chromium tetra t-butoxide are also known.[14]
Most chromium(I) compounds are obtained solely by oxidation of electron-rich, octahedral chromium(0) complexes. Other chromium(I) complexes contain cyclopentadienyl ligands. As verified by X-ray diffraction, a Cr-Cr quintuple bond (length 183.51(4) pm) has also been described.[15] Extremely bulky monodentate ligands stabilize this compound by shielding the quintuple bond from further reactions.
Notes
- ↑ Most common oxidation states of chromium are in bold. The right column lists a representative compound for each oxidation state.
See also
References
- ↑ Kotaś, J.; Stasicka, Z. (2000). "Chromium occurrence in the environment and methods of its speciation". Environmental Pollution 107 (3): 263–283. doi:10.1016/S0269-7491(99)00168-2. PMID 15092973.
- ↑ Puigdomenech, Ignasi Hydra/Medusa Chemical Equilibrium Database and Plotting Software (2004) KTH Royal Institute of Technology
- ↑ Clark, Jim. "Oxidation states (oxidation numbers)". https://www.chemguide.co.uk/inorganic/redox/oxidnstates.html.
- ↑ 4.0 4.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Theopold, Klaus H.; Kucharczyk, Robin R. (2011-12-15), Scott, Robert A., ed. (in en), Chromium: Organometallic Chemistry, John Wiley & Sons, Ltd, pp. eibc0042, doi:10.1002/9781119951438.eibc0042, ISBN 978-1-119-95143-8.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Holleman, Arnold F; Wiber, Egon; Wiberg, Nils (1985). "Chromium" (in de). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 1081–1095. ISBN 978-3-11-007511-3.
- ↑ Cotton, FA; Walton, RA (1993). Multiple Bonds Between Metal Atoms. Oxford: Oxford University Press. ISBN 978-0-19-855649-7. https://archive.org/details/multiplebondsbet0000cott.
- ↑ "Chromium(III) compounds". Commonwealth of Australia. http://www.npi.gov.au/resource/chromium-iii-compounds.
- ↑ Assfalg, M; Banci, L; Bertini, I; Bruschi, M; Michel, C; Giudici-Orticoni, M; Turano, P (31 July 2002). "NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c7". Protein Data Bank (1LM2). doi:10.2210/pdb1LM2/pdb. https://www.rcsb.org/structure/1lm2. Retrieved 8 November 2018.
- ↑ Luther, George W. (2016). "Introduction to Transition Metals". Inorganic Chemistry for Geochemistry & Environmental Sciences: Fundamentals & Applications. John Wiley & Sons. p. 244. ISBN 978-1118851371. https://books.google.com/books?id=Fz7hCgAAQBAJ&pg=PA244. Retrieved 2019-08-07.
- ↑ Gumerova, Nadiia I.; Roller, Alexander; Giester, Gerald; Krzystek, J.; Cano, Joan; Rompel, Annette (2020-02-19). "Incorporation of CrIII into a Keggin Polyoxometalate as a Chemical Strategy to Stabilize a Labile {CrIIIO4} Tetrahedral Conformation and Promote Unattended Single-Ion Magnet Properties". Journal of the American Chemical Society 142 (7): 3336–3339. doi:10.1021/jacs.9b12797. ISSN 0002-7863. PMID 31967803.
- ↑ Seppelt, Konrad (2015-01-28). "Molecular Hexafluorides" (in en). Chemical Reviews 115 (2): 1296–1306. doi:10.1021/cr5001783. ISSN 0009-2665. PMID 25418862.
- ↑ Haxhillazi, Gentiana (2003). Preparation, Structure and Vibrational Spectroscopy of Tetraperoxo Complexes of CrV+, VV+, NbV+ and TaV+ (PhD thesis). University of Siegen.
- ↑ Thaler, Eric G.; Rypdal, Kristin; Haaland, Arne; Caulton, Kenneth G. (1989-06-01). "Structure and reactivity of chromium(4+) tert-butoxide". Inorganic Chemistry 28 (12): 2431–2434. doi:10.1021/ic00311a035. ISSN 0020-1669. https://doi.org/10.1021/ic00311a035.
- ↑ Nguyen, T; Sutton, AD; Brynda, M; Fettinger, JC; Long, GJ; Power, PP (2005). "Synthesis of a stable compound with fivefold bonding between two chromium(I) centers". Science 310 (5749): 844–847. doi:10.1126/science.1116789. PMID 16179432. Bibcode: 2005Sci...310..844N.
 |