Chemistry:Chromium(II) oxide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
chromium(II) oxide
| |
| Identifiers | |
PubChem CID
|
|
| Properties | |
| CrO | |
| Molar mass | 67.996 g/mol |
| Appearance | black |
| Melting point | 300 °C (572 °F; 573 K) (decomposes) |
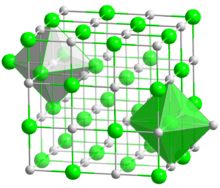
| Structure | |
| cubic, cF8 | |
| Fm3m, No. 225 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromium(II) oxide (CrO) is an inorganic compound composed of chromium and oxygen.[1] It is a black powder that crystallises in the rock salt structure.[2] Hypophosphites may reduce chromium(III) oxide to chromium(II) oxide:
- H3PO2 + 2 Cr2O3 → 4 CrO + H3PO4
It is readily oxidized by the atmosphere. CrO is basic, while CrO
3 is acidic, and Cr
2O
3 is amphoteric.[3]
CrO occurs in the spectra of luminous red novae, which occur when two stars collide. It is not known why red novae are the only objects that feature this molecule; one possible explanation is an as-yet-unknown nucleosynthesis process.[4]
See also
References
- ↑ Satish. Anand, Raj. Kumar (1989), Dictionary of Inorganic Chemistry, Anmol Publications, ISBN 978-81-7041-236-6, https://books.google.com/books?id=6yDA6nb5KCUC&dq=%22Chromium(II)+oxide%22&pg=PA65
- ↑ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN:0-12-352651-5
- ↑ Chemistry 7th edition, by Raymond Chang page 645 (problem 15.100)
- ↑ Kamiński, T.; Mason, E.; Tylenda, R.; Schmidt, M. R. (2015). "Post-outburst spectra of a stellar-merger remnant of V1309 Scorpii: From a twin of V838 Monocerotis to a clone of V4332 Sagittarii". Astronomy & Astrophysics 580: A34. doi:10.1051/0004-6361/201526212. Bibcode: 2015A&A...580A..34K.
 |

