Chemistry:Chromium(II) bromide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Chromium(II) bromide
| |
| Other names
Chromium dibromide, Chromium bromide, Chromous bromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CrBr2 | |
| Molar mass | 211.804 g·mol−1 |
| Appearance | White solid[1] |
| Density | 4.236 g/cm3[1] |
| Melting point | 842 °C (1,548 °F; 1,115 K)[1] |
| soluble, exothermal blue solution[1] | |
| Structure | |
| monoclinic[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
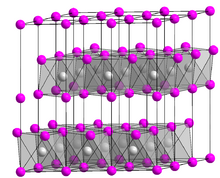
Chromium(II) bromide is the inorganic compound with the chemical formula CrBr2. Like many metal dihalides, CrBr2 adopts the "cadmium iodide structure" motif, i.e., it features sheets of octahedral Cr(II) centers interconnected by bridging bromide ligands. It is a white solid that dissolves in water to give blue solutions that are readily oxidized by air.
Synthesis and reactions
It can be prepared by reduction of chromium(III) bromide with hydrogen gas for 6–10 hours at 350-400 °C, cogenerating hydrogen bromide:[2]
- 2 CrBr3 + H2 → 2 CrBr2 + 2 HBr
Treatment of chromium powder with concentrated hydrobromic acid gives a blue hydrated chromium(II) bromide, which can be converted to a related acetonitrile complex.[3]
- Cr + n H2O + 2 HBr → CrBr2(H2O)n + H2
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Perry, Dale L. (2011). Handbook of Inorganic Compounds, Second Edition. Boca Raton, Florida: CRC Press. p. 120. ISBN 978-1-43981462-8. https://books.google.com/books?id=SFD30BvPBhoC. Retrieved 2014-01-10.
- ↑ Brauer, Georg (1965) (in de). Handbuch Der Präparativen Anorganischen Chemie. 2. Stuttgart; New York, New York: Ferdinand Enke Verlag; Academic Press, Inc.. p. 1341. ISBN 978-0-32316129-9. https://books.google.com/books?id=Pef47TK5NfkC. Retrieved 2014-01-10.
- ↑ Holah, David G.; Fackler, John P. (1967). "Chromium(II) Salts and Complexes". Inorganic Syntheses. 10. pp. 26–35. doi:10.1002/9780470132418.ch4. ISBN 9780470131695.
 |

