Chemistry:Chromium(III) boride
| Names | |
|---|---|
| IUPAC name
boranylidynechromium
| |
| Other names
Chromium monoboride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| CrB | |
| Molar mass | 62.81 g/mol |
| Appearance | silver, ceramic material |
| Density | 6.17 g/cm3 |
| Melting point | 1,950 to 2,050 °C (3,540 to 3,720 °F; 2,220 to 2,320 K) |
| insoluble | |
| Structure | |
| orthorhombic (space group Cmcm) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3[1] |
REL (Recommended)
|
TWA 0.5 mg/m3[1] |
IDLH (Immediate danger)
|
250 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromium(III) boride, also known as chromium monoboride (CrB), is an inorganic compound with the chemical formula CrB.[2] It is one of the six stable binary borides of chromium, which also include Cr2B, Cr5B3, Cr3B4, CrB2, and CrB4.[3] Like many other transition metal borides, it is extremely hard (21-23 GPa),[4][5] has high strength (690 MPa bending strength),[5] conducts heat and electricity as well as many metallic alloys,[4][6][7] and has a high melting point (~2100 °C).[8][3] Unlike pure chromium, CrB is known to be a paramagnetic, with a magnetic susceptibility that is only weakly dependent on temperature.[9][10] Due to these properties, among others, CrB has been considered as a candidate material for wear resistant coatings and high-temperature diffusion barriers.[citation needed]
It can be synthesized as powders by many methods including direct reaction of the constituent elemental powders,[11] self-propagating high-temperature synthesis (SHS),[5] borothermic reduction,[12][13] and molten salt growth.[14] Slow-cooling of molten aluminum solutions from high-temperatures has been used to grow large single crystals, with a maximum size of 0.6 mm x 0.6 mm x 8.3 mm.[4]
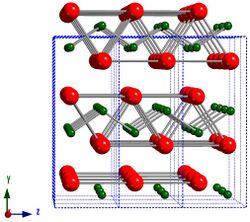
CrB has an orthorhombic crystal structure (space group Cmcm) that was first discovered in 1951,[15] and subsequently confirmed by later work using single crystals.[16] The crystal structure can be visualized as slabs face-sharing BCr6 trigonal prisms, in the ac-plane, that are stacked parallel to the <010> crystallographic direction. Similar to Cr3B4 and Cr2B3, the B atoms in the structure form covalent bonds with each other and are characterized by unidirectional B-B- chains parallel to the <001> crystallographic direction. The transition metal monoborides VB, NbB, TaB, and NiB have the same crystal structure. [citation needed]
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0141.html.
- ↑ Peshev, P.; Bliznakov, G.; Leyarovska, L. (1967). "On the preparation of some chromium, molybdenum and tungsten borides". Journal of the Less Common Metals 13 (2): 241. doi:10.1016/0022-5088(67)90188-9.
- ↑ 3.0 3.1 Liao, P. K.; Spear, K. E. (June 1986). "The B−Cr (Boron-Chromium) system". Bulletin of Alloy Phase Diagrams 7 (3): 232–237. doi:10.1007/BF02868996. ISSN 0197-0216.
- ↑ 4.0 4.1 4.2 Okada, Shigeru; Kudou, Kunio; Iizumi, Kiyokata; Kudaka, Katsuya; Higashi, Iwami; Lundström, Torsten (September 1996). "Single-crystal growth and properties of CrB, Cr3B4, Cr2B3 and CrB2 from high-temperature aluminum solutions". Journal of Crystal Growth 166 (1–4): 429–435. doi:10.1016/0022-0248(95)00890-X. Bibcode: 1996JCrGr.166..429O.
- ↑ 5.0 5.1 5.2 Hiroki, Yuji; Yoshinaka, Masaru; Hirota, Ken; Yamaguchi, Osamu (2003). "Hot Isostatic Pressing of CrB Prepared by Self-propagating High-temperature Synthesis". Journal of the Japan Society of Powder and Powder Metallurgy 50 (5): 367–371. doi:10.2497/jjspm.50.367. ISSN 0532-8799.
- ↑ L'vov, S. N.; Nemchenko, V. F.; Kislyi, P. S.; Verkhoglyadova, T. S.; Kosolapova, T. Ya. (1964). "The electrical properties of chromium borides, carbides, and nitrides". Soviet Powder Metallurgy and Metal Ceramics 1 (4): 243–247. doi:10.1007/BF00774426. ISSN 0038-5735.
- ↑ Ohishi, Yuji; Sugizaki, Mitsuyuki; Sun, Yifan; Muta, Hiroaki; Kurosaki, Ken (2019-03-22). "Thermophysical and mechanical properties of CrB and FeB". Journal of Nuclear Science and Technology 56 (9–10): 859–865. doi:10.1080/00223131.2019.1593893. ISSN 0022-3131.
- ↑ Kislyi, P. S.; L'vov, S. N.; Nemchenko, V. F.; Samsonov, G. V. (1964). "Physical properties of the boride phases of chromium". Soviet Powder Metallurgy and Metal Ceramics 1 (6): 441–443. doi:10.1007/BF00773921. ISSN 0038-5735.
- ↑ Guy, C.N. (1976). "The electronic properties of chromium borides". Journal of Physics and Chemistry of Solids 37 (11): 1005–1009. doi:10.1016/0022-3697(76)90123-2. Bibcode: 1976JPCS...37.1005G.
- ↑ Kota, Sankalp; Wang, Wenzhen; Lu, Jun; Natu, Varun; Opagiste, Christine; Ying, Guobing; Hultman, Lars; May, Steven J. et al. (October 2018). "Magnetic properties of Cr2AlB2, Cr3AlB4, and CrB powders". Journal of Alloys and Compounds 767: 474–482. doi:10.1016/j.jallcom.2018.07.031.
- ↑ Lundquist, N.; Myers, H. P.; Westin, R. (July 1962). "The paramagnetic properties of the monoborides of V, Cr, Mn, Fe, Co and Ni". Philosophical Magazine 7 (79): 1187–1195. doi:10.1080/14786436208209119. ISSN 0031-8086. Bibcode: 1962PMag....7.1187L.
- ↑ Okada, Shigeru; Iizumi, Kiyokata; Ogino, Tomoyuki; Kudaka, Katsuya; Kudou, Kunio (1996). "Preparation of CrB Single Crystals by the Reaction between Chromium Oxide and Amorphous Boron Powders.". Nippon Kagaku Kaishi 1996 (3): 260–263. doi:10.1246/nikkashi.1996.260. ISSN 2185-0925. http://joi.jlc.jst.go.jp/JST.Journalarchive/nikkashi1972/1996.260?from=CrossRef.
- ↑ Iizumi, Kiyokata; Kudaka, Katsuya; Okada, Shigeru (1998). "Synthesis of Chromium Borides by Solid-State Reaction between Chromium Oxide (III) and Amorphous Boron Powders". Journal of the Ceramic Society of Japan 106 (1237): 931–934. doi:10.2109/jcersj.106.931. ISSN 1882-1022.
- ↑ Cao, Weixiao; Wei, Ya'nan; Meng, Xin; Ji, Yuexia; Ran, Songlin (2017-04-13). "A general method towards transition metal monoboride nanopowders". International Journal of Materials Research 108 (4): 335–338. doi:10.3139/146.111484. ISSN 1862-5282.
- ↑ Frueh, A. J. (1951-01-01). "Confirmation of the structure of chromium boride, CrB". Acta Crystallographica 4 (1): 66–67. doi:10.1107/S0365110X51000118. ISSN 0365-110X.
- ↑ Okada, Shigeru; Atoda, Tetsuzo; Higashi, Iwami (May 1987). "Structural investigation of Cr2B3, Cr3B4, and CrB by single-crystal diffractometry". Journal of Solid State Chemistry 68 (1): 61–67. doi:10.1016/0022-4596(87)90285-4. Bibcode: 1987JSSCh..68...61O.
 |