Chemistry:Chromyl fluoride
| |
| Names | |
|---|---|
| IUPAC name
Difluoro(dioxo)chromium
| |
| Other names
Chromyl Fluoride, Chromium Difluoride Dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
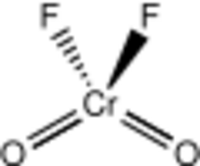
| CrO 2F 2 | |
| Molar mass | 121.991 g·mol−1 |
| Appearance | Violet-red crystals |
| Melting point | 31.6 °C (88.9 °F; 304.8 K) |
| Boiling point | 30 °C (86 °F; 303 K) Sublimes |
| Structure | |
| monoclinic | |
| P21/c, No. 14 | |
| C2v | |
Formula units (Z)
|
4 |
| Hazards | |
| Main hazards | Oxidant |
| Related compounds | |
Related compounds
|
chromyl chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromyl fluoride is an inorganic compound with the formula CrO
2F
2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.[1]
Structure
The liquid and gaseous CrO
2F
2 have a tetrahedral geometry with C2v symmetry, much like chromyl chloride.[2] Chromyl fluoride dimerizes via fluoride bridges (as O
2Cr(μ-F)
4CrO
2) in the solid state, crystallizing in the P21/c space group with Z = 4. The Cr=O bond lengths are about 157 pm, and the Cr–F bond lengths are 181.7, 186.7, and 209.4 pm. Chromium resides in a distorted octahedral position with a coordination number of 6.[3]
History and preparation
Pure chromyl fluoride was first isolated in 1952 as reported by Alfred Engelbrecht and Aristid von Grosse.[4] It was first observed as red vapor in the early 19th century upon heating a mixture of fluorspar (CaF
2), chromates, and sulfuric acid. These red vapors were initially thought to be CrF
6, although some chemists assumed a CrO
2F
2 structure analogous to CrO
2Cl
2.[4] The first moderately successful synthesis of chromyl fluoride was reported by Fredenhagen who examined the reaction of hydrogen fluoride with alkali chromates. A later attempt saw von Wartenberg prepare impure CrO
2F
2 by treating chromyl chloride with elemental fluorine.[5] Another attempt was made by Wiechert, who treated HF with dichromate, yielding impure liquid CrO
2F
2 at −40 °C.
Engelbrecht and von Grosse's synthesis of CrO
2F
2, and most successive syntheses, involve treating chromium trioxide with a fluorinating agent:[4]
- CrO
3 + 2 HF → CrO
2F
2 + H
2O
The reaction is reversible, as water will readily hydrolyze CrO
2F
2 back to CrO
3.
The approach published by Georg Brauer in the Handbook of Preparative Inorganic Chemistry[6] drew on von Wartenberg's approach[5] of direct fluoridation:
- CrO
2Cl
2 + F
2 → CrO
2F
2 + Cl
2
Other methods include treatment with chlorine fluoride, carbonyl fluoride, or some metal hexafluorides:
- CrO
3 + 2 ClF → CrO
2F
2 + Cl
2 + O
2 - CrO
3 + COF
2 → CrO
2F
2 + CO
2 - CrO
3 + MF
6 → CrO
2F
2 + MOF
4 (M = Mo, W)
The last method involving the fluorides of tungsten and molybdenum are reported by Green and Gard to be very simple and effective routes to large quantities of pure CrO
2F
2.[1] They reported 100% yield when the reactions were conducted at 120 °C. As expected from the relative reactivities of MoF
6 and WF
6, the molybdenum reaction proceeded more readily than did the tungsten.[7]
Reactions
Chromyl fluoride is a strong oxidizing agent capable of converting hydrocarbons to ketones and carboxylic acids. It can also be used as a reagent in the preparation of other chromyl compounds.[1] Like some other fluoride compounds, CrO
2F
2 reacts with glass and quartz, so silicon-free plastics or metal containers are required for handling the compound. Its oxidizing power in inorganic systems has also been explored.[8] Chromyl fluoride can exchange fluorine atoms with metal oxides.
- CrO
2F
2 + MO → MF
2 + CrO
3
Chromyl fluoride will also convert the oxides of boron and silicon to the fluorides.[8]
Chromyl fluoride reacts with alkali and alkaline earth metal fluorides in perfluoroheptane (solvent) to produce orange-colored fluorochromates:[8]
- CrO
2F
2 + 2 MF → M
2[CrO
2F
4]
Chromyl fluoride also reacts with Lewis acids, drawing carboxylate ligands from organic acid anhydrides and producing an acyl fluoride byproduct:[8]
- CrO
2F
2 + 2 (CF
3CO)
2O → (CF
3COO)
2CrO
2 + 2 CF
3COF
Chromyl fluoride forms adducts with weak bases NO, NO
2, and SO
2.
References
- ↑ 1.0 1.1 1.2 Gard, G. L. (1986) "Chromium Difluoride Dioxide (Chromyl Fluoride)," Inorg. Synth., 24, 67-69, doi:10.1002/9780470132555.ch20.
- ↑ Hobbs, W. E. (1958) "Infrared Absorption Spectra of Chromyl Fluoride and Chromyl Chloride," J. Chem. Phys. 28(6), 1220-1222, doi:10.1063/1.1744372.
- ↑ Supeł, J.; Abram, U.; Hagenbach, A.; Seppelt, K. (2007) "Technetium Fluoride Trioxide, TcO3F, Preparation and Properties." Inorg. Chem., 46(14), 5591–5595, doi:10.1021/ic070333y.
- ↑ 4.0 4.1 4.2 Engelbrecht, A.; von Grosse, A. (1952) "Pure Chromyl Fluoride," J. Am. Chem. Soc. 74(21), 5262–5264, doi:10.1021/ja01141a007.
- ↑ 5.0 5.1 von Wartenberg, H. (1941) "Über höhere Chromfluoride (CrF4, CrF5 und CrO2F2)" [About higher chromium fluorides (CrF4, CrF5 and CrO2F2)], Z. Anorg. Allg. Chem. [in German], 247(1-2), 135–146, doi:10.1002/zaac.19412470112.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBrauer - ↑ Green, P. J.; Gard, G. L. (1977) "Chemistry of Chromyl Fluoride. 5. New Preparative routes to CrO2F2," Inorg. Chem. 16(5), 1243–1245, doi:10.1021/ic50171a055.
- ↑ 8.0 8.1 8.2 8.3 Brown, S. D.; Green, P.J.; Gard, G.L. (1975) "The Chemistry of Chromyl Fluoride III: Reactions with Inorganic Systems," J. Fluorine Chem. 5(3), 203-219, doi:10.1016/S0022-1139(00)82482-3.
 |

