Chemistry:Diphenylacetylene
| |
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1′-Ethynediyldibenzene | |
| Other names
Tolane
1,2-Diphenylethyne Diphenylethyne 2-Phenylethynylbenzene Tolan | |
| Identifiers | |
3D model (JSmol)
|
|
| 606478 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H10 | |
| Molar mass | 178.234 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.136 g cm−3[1] |
| Melting point | 62.5 °C (144.5 °F; 335.6 K) |
| Boiling point | 170 °C (338 °F; 443 K) at 19 mmHg |
| Insoluble | |
| Structure | |
| 0 D | |
| Hazards | |
| Safety data sheet | Fisher Scientific MSDS |
| Related compounds | |
Related compounds
|
But-2-yne Dimethyl acetylenedicarboxylate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
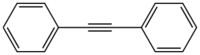
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.
Preparation and structure
In one preparation for this compound, benzil is condensed with hydrazine to give the bis(hydrazone), which is oxidized with mercury(II) oxide.[2] Alternatively stilbene is brominated, and the resulting dibromodiphenylethane is subjected to dehydrohalogenation.[3] Yet another method involves the coupling of iodobenzene and the copper salt of phenylacetylene in the Castro-Stephens coupling. The related Sonogashira coupling involves the coupling of iodobenzene and phenylacetylene.
Diphenylacetylene is a planar molecule. The central C≡C distance is 119.8 picometers.[1]
Derivatives
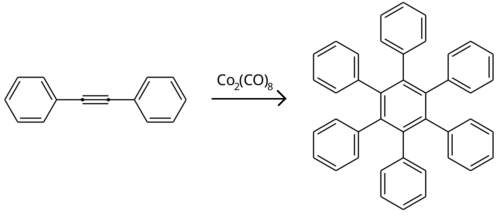
Reaction of diphenylacetylene with tetraphenylcyclopentadienone results in the formation of hexaphenylbenzene in a Diels–Alder reaction.[4]
Dicobalt octacarbonyl catalyzes alkyne trimerisation of diphenylacetylene to form hexaphenylbenzene.[5]
Reaction of Ph2C2 with benzal chloride in the presence of potassium t-butoxide affords 3-tert-butoxy-1,2,3-triphenylcyclopropene, which converts to 1,2,3-triphenylcyclopropenium bromide after the elimination of tert-butoxide.[6]
References
- ↑ 1.0 1.1 Mavridis, A.; Moustakali-Mavridis, I. (1977). "A Reinvestigation of Tolane". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 33 (11): 3612–3615. doi:10.1107/S0567740877011674.
- ↑ Cope, A. C.; Smith, D. S.; Cotter, R. J. (1954). "Diphenylacetylene". Organic Syntheses 34: 42. doi:10.15227/orgsyn.034.0042.
- ↑ Lee Irvin Smith; M. M. Falkof (1942). "Diphenylacetylene". Organic Syntheses 22: 50. doi:10.15227/orgsyn.022.0050.
- ↑ Fieser, L. F. (1966). "Hexaphenylbenzene". Organic Syntheses 46: 44. doi:10.15227/orgsyn.046.0044.
- ↑ Vij, V.; Bhalla, V.; Kumar, M. (8 August 2016). "Hexaarylbenzene: Evolution of Properties and Applications of Multitalented Scaffold". Chemical Reviews 116 (16): 9565–9627. doi:10.1021/acs.chemrev.6b00144.
- ↑ Xu, Ruo; Breslow, Ronald (1997). "1,2,3-Triphenylcyclopropenium Bromide". Organic Syntheses 74: 72. doi:10.15227/orgsyn.074.0072.
 |