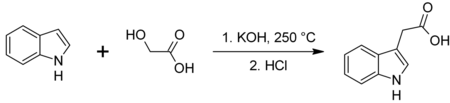Chemistry:Indole-3-acetic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1H-Indol-3-yl)acetic acid | |
| Other names
Indole-3-acetic acid,
indolylacetic acid, 1H-Indole-3-acetic acid, indoleacetic acid, heteroauxin, IAA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H9NO2 | |
| Molar mass | 175.187 g·mol−1 |
| Appearance | White solid |
| Melting point | 168 to 170 °C (334 to 338 °F; 441 to 443 K) |
| insoluble in water. Soluble in ethanol to 50mg/ml | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
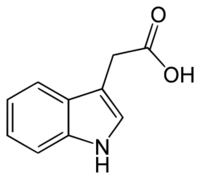
Indole-3-acetic acid (IAA, 3-IAA) is the most common naturally occurring plant hormone of the auxin class. It is the best known of the auxins, and has been the subject of extensive studies by plant physiologists.[1] IAA is a derivative of indole, containing a carboxymethyl substituent. It is a colorless solid that is soluble in polar organic solvents.
Biosynthesis
IAA is predominantly produced in cells of the apex (bud) and very young leaves of a plant. Plants can synthesize IAA by several independent biosynthetic pathways. Four of them start from tryptophan, but there is also a biosynthetic pathway independent of tryptophan.[2] Plants mainly produce IAA from tryptophan through indole-3-pyruvic acid.[3][4] IAA is also produced from tryptophan through indole-3-acetaldoxime in Arabidopsis thaliana.[5]
In rats, IAA is a product of both endogenous and colonic microbial metabolism from dietary tryptophan along with tryptophol. This was first observed in rats infected by Trypanosoma brucei gambiense.[6] A 2015 experiment showed that a high-tryptophan diet can decrease serum levels of IAA in mice, but that in humans, protein consumption has no reliably predictable effect on plasma IAA levels.[7] Human cells have been known to produce IAA in vitro since the 1950s,[8] and the critical biosynthesis gene IL4I1 has been identified.[9][10]
Biological effects
As all auxins, IAA has many different effects, such as inducing cell elongation and cell division with all subsequent results for plant growth and development. On a larger scale, IAA serves as signaling molecule necessary for development of plant organs and coordination of growth.
Plant gene regulation
IAA enters the plant cell nucleus and binds to a protein complex composed of a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3), resulting in ubiquitination of Aux/IAA proteins with increased speed.[11] Aux/IAA proteins bind to auxin response factor (ARF) proteins, forming a heterodimer, suppressing ARF activity.[12] In 1997 it was described how ARFs bind to auxin-response gene elements in promoters of auxin regulated genes, generally activating transcription of that gene when an Aux/IAA protein is not bound.[13]
IAA inhibits the photorespiratory-dependent cell death in photorespiratory catalase mutants. This suggests a role for auxin signalling in stress tolerance.[14]
Bacterial physiology
IAA production is widespread among environmental bacteria that inhabit soils, waters, but also plant and animal hosts. Distribution and substrate specificity of the involved enzymes suggests these pathways play a role beyond plant-microbe interactions.[15] Enterobacter cloacae can produce IAA, from aromatic and branched-chain amino acids.[16]
Fungal symbiosis
Fungi can form a fungal mantle around roots of perennial plants called ectomycorrhiza. A fungus specific to spruce called Tricholoma vaccinum was shown to produce IAA from tryptophan and excrete it from its hyphae. This induced branching in cultures, and enhanced Hartig net formation. The fungus uses a multidrug and toxic extrusion (MATE) transporter Mte1.[17] Research into IAA-producing fungi to promote plant growth and protection in sustainable agriculture is underway.[18]
Skatole biosynthesis
Skatole, the odorant in feces, is produced from tryptophan via indoleacetic acid. Decarboxylation gives the methylindole.[19][20]
Synthesis
Chemically, it can be synthesized by the reaction of indole with glycolic acid in the presence of base at 250 °C:[21]
Alternatively the compound has been synthesized by Fischer indole synthesis using glutamic acid and phenylhydrazine.[22] Glutamic acid was converted to the necessary aldehyde via Strecker degradation.
Many methods for its synthesis have been developed since its original synthesis from indole-3-acetonitrile.[23]
History and synthetic analogs
William Gladstone Tempelman studied substances for growth promotion at Imperial Chemical Industries Ltd. After 7 years of research he changed the direction of his study to try the same substances at high concentrations in order to stop plant growth. In 1940 he published his finding that IAA killed broadleaf plants within a cereal field.[24]
The search for an acid with a longer half life, i.e. a metabolically and environmentally more stable compound led to 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), both phenoxy herbicides and analogs of IAA. Robert Pokorny an industrial chemist for the C.B. Dolge Company in Westport, Connecticut published their synthesis in 1941.[25] When sprayed on broad-leaf dicot plants, they induce rapid, uncontrolled growth, eventually killing them. First introduced in 1946, these herbicides were in widespread use in agriculture by the middle of the 1950s.[citation needed]
Other less expensive synthetic auxin analogs on the market for use in horticulture are indole-3-butyric acid (IBA) and 1-naphthaleneacetic acid (NAA).[26]
Mammalian toxicity/health effects
Little research has been conducted on the effects of IAA on humans and toxicity data are limited. No data on human carcinogenic, teratogenic, or developmental effects have been created.
IAA is listed in its MSDS as mutagenic to mammalian somatic cells, and possibly carcinogenic based on animal data. It may cause adverse reproductive effects (fetotoxicity) and birth defects based on animal data. No human data as of 2008.[27] It is listed as a potential skin, eye, and respiratory irritant, and users are warned not to ingest it. Protocols for ingestion, inhalation, and skin/eye exposure are standard for moderately poisonous compounds and include thorough rinsing in the case of skin and eyes, fresh air in the case of inhalation, and immediately contacting a physician in all cases to determine the best course of action and not to induce vomiting when of ingested. The NFPA 704 health hazard rating for IAA is 2, which denotes a risk of temporary incapacitation with intense or prolonged, but not chronic exposure, and a possibility of residual injury.[28] IAA is a direct ligand of the aryl hydrocarbon receptor,[29] and IAA treatment of mice indicate liver-protective effects in a model of non-alcoholic fatty liver disease.[30] Humans typically have relatively high levels of IAA in their serum (~1µM), but this can be increased further in certain disease conditions and can be a poor prognostic marker for cardiovascular health.[31] Whether this IAA originates from endogenous biosynthesis via IL4I1 or gut microbiota is unknown. A 2021 study found that normal mice had an average of 3.7 times as much IAA in their feces compared to germ-free mice, suggesting that the mammalian microbiome contributes significantly to the overall circulating amount. [32]
Developmental toxicity
IAA produces microcephaly in rats during the early stage of cerebral cortex development. IAA treatment of pregnant rats, at a dose of 1 gram per kg of body weight per day, decreased the locomotor activities of rat embryos/fetuses; treatment with IAA and analog 1(methyl)-IAA resulted in apoptosis of neuroepithelial cell and significantly decreased brain sizes relative to body weight in embryonic rats.[33]
Immunotoxin
IAA is an apoptosis-inducing ligand in mammals. As of 2010, the signal transduction pathways are as follows: IAA/HRP activates p38 mitogen-activated protein kinases and c-Jun N-terminal kinases. It induces caspase-8 and caspase-9, which results in caspase-3 activation and poly(adp-ribose) polymerases cleavage.[34]
In 2002 it had been hypothesized that IAA coupled with horseradish peroxidase (HRP) could be used in targeted cancer therapy. Radical-IAA molecules would attach to cells marked by HRP and HRP reactive cells would be selectively killed.[35] In 2010 in vitro experiments proved this concept of IAA as an immunotoxin when used in preclinical studies of targeted cancer therapy, as it induced apoptosis in bladder[34] and in hematological malignancies.[36]
References
- ↑ Simon, Sibu; Petrášek, Jan (2011). "Why plants need more than one type of auxin". Plant Science 180 (3): 454–60. doi:10.1016/j.plantsci.2010.12.007. PMID 21421392. https://zenodo.org/record/894396.
- ↑ Zhao, Yunde (2010). "Auxin Biosynthesis and Its Role in Plant Development". Annual Review of Plant Biology 61: 49–64. doi:10.1146/annurev-arplant-042809-112308. PMID 20192736.
- ↑ Mashiguchi, Kiyoshi; Tanaka, Keita; Sakai, Tatsuya; Sugawara, Satoko; Kawaide, Hiroshi; Natsume, Masahiro; Hanada, Atsushi; Yaeno, Takashi et al. (2011). "The main auxin biosynthesis pathway in Arabidopsis". Proceedings of the National Academy of Sciences 108 (45): 18512–7. doi:10.1073/pnas.1108434108. PMID 22025724. Bibcode: 2011PNAS..10818512M.
- ↑ Won, Christina; Shen, Xiangling; Mashiguchi, Kiyoshi; Zheng, Zuyu; Dai, Xinhua; Cheng, Youfa; Kasahara, Hiroyuki; Kamiya, Yuji et al. (2011). "Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis". Proceedings of the National Academy of Sciences 108 (45): 18518–23. doi:10.1073/pnas.1108436108. PMID 22025721. Bibcode: 2011PNAS..10818518W.
- ↑ Sugawara, Satoko; Hishiyama, Shojiro; Jikumaru, Yusuke; Hanada, Atsushi; Nishimura, Takeshi; Koshiba, Tomokazu; Zhao, Yunde; Kamiya, Yuji et al. (2009). "Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis". Proceedings of the National Academy of Sciences 106 (13): 5430–5. doi:10.1073/pnas.0811226106. PMID 19279202. Bibcode: 2009PNAS..106.5430S.
- ↑ Howard Stibbs Henry; Richard Seed John (1975). "Short-Term Metabolism of [14C]Tryptophan in Rats Infected with Trypanosoma brucei gambiense". J Infect Dis 131 (4): 459–462. doi:10.1093/infdis/131.4.459. PMID 1117200.
- ↑ "The Influence of Dietary Protein Intake on Mammalian Tryptophan and Phenolic Metabolites". PLOS ONE 10 (10): e0140820. Oct 2015. doi:10.1371/journal.pone.0140820. PMID 26469515. Bibcode: 2015PLoSO..1040820P.
- ↑ Weissbach, H.; King, W.; Sjoerdsma, A.; Udenfriend, S. (January 1959). "Formation of indole-3-acetic acid and tryptamine in animals: a method for estimation of indole-3-acetic acid in tissues". The Journal of Biological Chemistry 234 (1): 81–86. doi:10.1016/S0021-9258(18)70339-6. ISSN 0021-9258. PMID 13610897.
- ↑ Zhang, Xia; Gan, Min; Li, Jingyun; Li, Hui; Su, Meicheng; Tan, Dongfei; Wang, Shaolei; Jia, Man et al. (2020-08-31). "An endogenous indole pyruvate pathway for tryptophan metabolism mediated by IL4I1". Journal of Agricultural and Food Chemistry 68 (39): 10678–10684. doi:10.1021/acs.jafc.0c03735. ISSN 1520-5118. PMID 32866000.
- ↑ Sadik, Ahmed; Somarribas Patterson, Luis F.; Öztürk, Selcen; Mohapatra, Soumya R.; Panitz, Verena; Secker, Philipp F.; Pfänder, Pauline; Loth, Stefanie et al. (2020-08-17). "IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression". Cell 182 (5): 1252–1270.e34. doi:10.1016/j.cell.2020.07.038. ISSN 1097-4172. PMID 32818467.
- ↑ Pekker, MD; Deshaies, RJ (2005). "Function and regulation of cullin-RING ubiquitin ligases.". Plant Cell 6 (1): 9–20. doi:10.1038/nrm1547. PMID 15688063. https://authors.library.caltech.edu/55905/2/nrm1547-S1.pdf.
- ↑ Tiwari, SB; Hagen, G; Guilfoyle, TJ (2004). "Aux/IAA proteins contain a potent transcriptional repression domain.". Plant Cell 16 (2): 533–43. doi:10.1105/tpc.017384. PMID 14742873.
- ↑ Ulmasov, T; Hagen, G; Guilfoyle, TJ (1997). "ARF1, a transcription factor that binds to auxin response elements.". Science 276 (5320): 1865–68. doi:10.1126/science.276.5320.1865. PMID 9188533.
- ↑ "Activation of auxin signalling counteracts photorespiratory H2O2-dependent cell death". Plant Cell Environ 38 (2): 253–65. Feb 2015. doi:10.1111/pce.12250. PMID 26317137.
- ↑ "Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria". Crit Rev Microbiol 39 (4): 395–415. Nov 2013. doi:10.3109/1040841X.2012.716819. PMID 22978761.
- ↑ "Regulation of indole-3-acetic acid biosynthesis by branched-chain amino acids in Enterobacter cloacae UW5". FEMS Microbiol Lett 362 (18): fnv153. Sep 2015. doi:10.1093/femsle/fnv153. PMID 26347301.
- ↑ "Biosynthesis and Secretion of Indole-3-Acetic Acid and Its Morphological Effects on Tricholoma vaccinum-Spruce Ectomycorrhiza". Appl Environ Microbiol 81 (20): 7003–11. Oct 2015. doi:10.1128/AEM.01991-15. PMID 26231639. Bibcode: 2015ApEnM..81.7003K.
- ↑ "Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms". Plant Signal Behav 10 (8): e1048052. Aug 2015. doi:10.1080/15592324.2015.1048052. PMID 26179718.
- ↑ Whitehead, T. R.; Price, N. P.; Drake, H. L.; Cotta, M. A. (25 January 2008). "Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure". Applied and Environmental Microbiology 74 (6): 1950–3. doi:10.1128/AEM.02458-07. PMID 18223109. Bibcode: 2008ApEnM..74.1950W.
- ↑ Yokoyama, M. T.; Carlson, J. R. (1979). "Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole". The American Journal of Clinical Nutrition 32 (1): 173–178. doi:10.1093/ajcn/32.1.173. PMID 367144.
- ↑ Johnson, Herbert E.; Crosby, Donald G. (1964). "Indole-3-acetic Acid". Organic Syntheses 44: 64. http://www.orgsyn.org/demo.aspx?prep=CV5P0654.; Collective Volume, 5, pp. 654
- ↑ Fox, Sidney W.; Bullock, Milon W. (1951). "Synthesis of Indoleacetic Acid from Glutamic Acid and a Proposed Mechanism for the Conversion". Journal of the American Chemical Society 73 (6): 2754–2755. doi:10.1021/ja01150a094.
- ↑ Majima, Rikō; Hoshino, Toshio (1925). "Synthetische Versuche in der Indol-Gruppe, VI.: Eine neue Synthese von β-Indolyl-alkylaminen". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 58 (9): 2042–6. doi:10.1002/cber.19250580917.
- ↑ Templeman W. G.; Marmoy C. J. (2008). "The effect upon the growth of plants of watering with solutions of plant-growth substances and of seed dressings containing these materials". Annals of Applied Biology 27 (4): 453–471. doi:10.1111/j.1744-7348.1940.tb07517.x.
- ↑ Pokorny Robert (1941). "New Compounds. Some Chlorophenoxyacetic Acids". J. Am. Chem. Soc. 63 (6): 1768. doi:10.1021/ja01851a601.
- ↑ "PGR Planofix - Crop Science India". https://www.cropscience.bayer.in/Products-H/Brands/Crop-Protection/PGR-Planofix.
- ↑ "1H-Indole-3-acetic acid" Registry of Toxic Effects of Chemical Substances (RTECS). Page last updated:November 8, 2017.
- ↑ "Indole-3-Acetic Acid: Material Safety Data Sheet." November 2008.
- ↑ Miller, Charles A. (1997-12-26). "Expression of the Human Aryl Hydrocarbon Receptor Complex in Yeast ACTIVATION OF TRANSCRIPTION BY INDOLE COMPOUNDS". Journal of Biological Chemistry 272 (52): 32824–32829. doi:10.1074/jbc.272.52.32824. ISSN 1083-351X. PMID 9407059. http://www.jbc.org/content/272/52/32824. Retrieved 2020-01-08.
- ↑ Ji, Yun; Gao, Yuan; Chen, Hong; Yin, Yue; Zhang, Weizhen (2019-09-03). "Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress". Nutrients 11 (9): 2062. doi:10.3390/nu11092062. ISSN 2072-6643. PMID 31484323.
- ↑ Dou, Laetitia; Sallée, Marion; Cerini, Claire; Poitevin, Stéphane; Gondouin, Bertrand; Jourde-Chiche, Noemie; Fallague, Karim; Brunet, Philippe et al. (April 2015). "The cardiovascular effect of the uremic solute indole-3 acetic acid". Journal of the American Society of Nephrology 26 (4): 876–887. doi:10.1681/ASN.2013121283. ISSN 1533-3450. PMID 25145928.
- ↑ Lai, Yunjia; Liu, Chih-Wei; Yang, Yifei; Hsiao, Yun-Chung; Ru, Hongyu; Lu, Kun (2021). "High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice.". Nature Communications 12 (6000): 6000. doi:10.1038/s41467-021-26209-8. PMID 34667167. Bibcode: 2021NatCo..12.6000L.
- ↑ Furukawa, Satoshi; Usuda, Koji; Abe, Masayoshi; Ogawa, Izumi (2005). "Effect of Indole-3-Acetic Acid Derivatives on Neuroepithelium in Rat Embryos". The Journal of Toxicological Sciences 30 (3): 165–74. doi:10.2131/jts.30.165. PMID 16141651.
- ↑ 34.0 34.1 "Indole-3-acetic acid/horseradish peroxidase induces apoptosis in TCCSUP human urinary bladder carcinoma cells". Pharmazie 65 (2): 122–6. 2010. PMID 20225657.
- ↑ Wardman P (2002). "Indole-3-acetic acids and horseradish peroxidase: a new prodrug/enzyme combination for targeted cancer therapy". Curr. Pharm. Des. 8 (15): 1363–74. doi:10.2174/1381612023394610. PMID 12052213.
- ↑ "Antibody-targeted horseradish peroxidase associated with indole-3-acetic acid induces apoptosis in vitro in hematological malignancies". Leuk. Res. 35 (5): 657–62. 2011. doi:10.1016/j.leukres.2010.11.025. PMID 21168913. cited in: "Immunotoxins for leukemia". Blood 123 (16): 2470–7. 2014. doi:10.1182/blood-2014-01-492256. PMID 24578503.
 |