Chemistry:Minor actinide
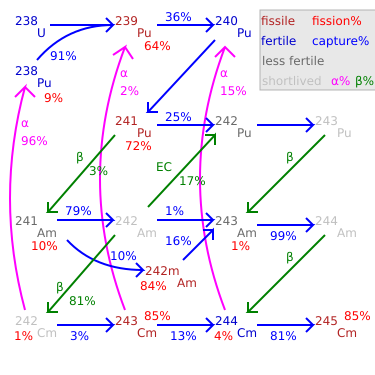
Fission percentage is 100 minus shown percentages.
Total rate of transmutation varies greatly by nuclide.
245Cm–248Cm are long-lived with negligible decay.
A minor actinide is an actinide, other than uranium or plutonium, found in spent nuclear fuel. The minor actinides include neptunium (element 93), americium (element 95), curium (element 96), berkelium (element 97), californium (element 98), einsteinium (element 99), and fermium (element 100).[2] The most important isotopes of these elements in spent nuclear fuel are neptunium-237, americium-241, americium-243, curium-242 through -248, and californium-249 through -252.
Plutonium and the minor actinides will be responsible for the bulk of the radiotoxicity and heat generation of spent nuclear fuel in the long term (300 to 20,000 years in the future).[3]
The plutonium from a power reactor tends to have a greater amount of plutonium-241 than the plutonium generated by the lower burnup operations designed to create weapons-grade plutonium. Because the reactor-grade plutonium contains so much 241Pu, the presence of 241Am makes the plutonium less suitable for making a nuclear weapon. The ingrowth of americium in plutonium is one of the methods for identifying the origin of an unknown sample of plutonium and the time since it was last separated chemically from the americium.
Americium is commonly used in industry as both an alpha particle source and as a low photon-energy gamma radiation source. For example, it is commonly used in smoke detectors. Americium can be formed by neutron capture of 239Pu and 240Pu, forming 241Pu which then beta decays to 241Am.[4] In general, as the energy of the neutrons increases, the ratio of the fission cross section to the neutron capture cross section changes in favour of fission. Hence, if MOX is used in a thermal reactor such as a boiling water reactor (BWR) or pressurized water reactor (PWR) then more americium can be expected to be found in the spent fuel than in that from a fast neutron reactor.[5]
Some of the minor actinides have been found in fallout from bomb tests. See Actinides in the environment for details.
| Isotope | Fraction | DLWR | Dfast | Dsuperthermal |
|---|---|---|---|---|
| 237Np | 0.0539 | 1.12 | −0.59 | −0.46 |
| 238Pu | 0.0364 | 0.17 | −1.36 | −0.13 |
| 239Pu | 0.451 | −0.67 | −1.46 | −1.07 |
| 240Pu | 0.206 | 0.44 | −0.96 | 0.14 |
| 241Pu | 0.121 | −0.56 | −1.24 | −0.86 |
| 242Pu | 0.0813 | 1.76 | −0.44 | 1.12 |
| 241Am | 0.0242 | 1.12 | −0.62 | −0.54 |
| 242mAm | 0.000088 | 0.15 | −1.36 | −1.53 |
| 243Am | 0.0179 | 0.82 | −0.60 | 0.21 |
| 243Cm | 0.00011 | −1.90 | −2.13 | −1.63 |
| 244Cm | 0.00765 | −0.15 | −1.39 | −0.48 |
| 245Cm | 0.000638 | −1.48 | −2.51 | −1.37 |
| Weighted sum | −0.03 | −1.16 | −0.51 | |
References
- ↑ Sasahara, Akihiro; Matsumura, Tetsuo; Nicolaou, Giorgos; Papaioannou, Dimitri (April 2004). "Neutron and Gamma Ray Source Evaluation of LWR High Burn-up UO2 and MOX Spent Fuels". Journal of Nuclear Science and Technology 41 (4): 448–456. doi:10.3327/jnst.41.448.
- ↑ Moyer, Bruce A. (2009). Ion Exchange and Solvent Extraction: A Series of Advances, Volume 19. CRC Press. pp. 120. ISBN 9781420059700. https://books.google.com/books?id=NTgjUaLZiDsC&pg=PA120.
- ↑ Stacey, Weston M. (2007). Nuclear Reactor Physics. John Wiley & Sons. pp. 240. ISBN 9783527406791. https://books.google.com/books?id=y1UgcgVSXSkC&pg=PA240.
- ↑ Raj, Gurdeep (2008). Advanced Inorganic Chemistry Vol-1, 31st ed.. Krishna Prakashan Media. pp. 356. ISBN 9788187224037. https://books.google.com/books?id=0uwDTrxyaB8C&pg=PA356.
- ↑ Berthou, V. (2003). "Transmutation characteristics in thermal and fast neutron spectra: application to americium". Journal of Nuclear Materials 320 (1–2): 156–162. doi:10.1016/S0022-3115(03)00183-1. Bibcode: 2003JNuM..320..156B. http://nucleonica.com/TC/TC0406/relevant_papers/Transmutation_characteristics.pdf. Retrieved 2013-03-31.
- ↑ Etienne Parent (2003). "Nuclear Fuel Cycles for Mid-Century Deployment". MIT. pp. 104. http://dspace.mit.edu/bitstream/handle/1721.1/17027/54495851.pdf.
 |

