Biology:Actinidain
| actinidain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
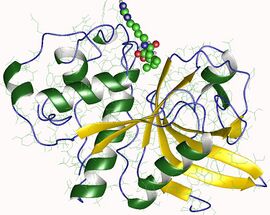 Biological assembly image of actinidain from Actinidia chinensis. From PDB: 1AEC. | |||||||||
| Identifiers | |||||||||
| EC number | 3.4.22.14 | ||||||||
| CAS number | 39279-27-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Actinidain (EC 3.4.22.14, actinidin, Actinidia anionic protease, proteinase A2 of Actinidia chinensis) is a type of cysteine protease enzyme found in fruits including kiwifruit (genus Actinidia), pineapple, mango, banana, figs, and papaya. This enzyme is part of the peptidase C1 family of papain-like proteases.[1][2][3][4]
As a known allergen in kiwifruit,[5] the enzyme is under preliminary research for its effect on tight junction proteins of intestinal epithelial cells.[6][7]
Actinidain is commercially useful as a meat tenderiser[8][9] and in coagulating milk for dairy products, like yogurt and cheese.[10] The denaturation temperature of actinidain is 60 °C (140 °F), lower than that of similar meat tenderising enzymes bromelain from pineapple and papain from papaya.[11]
History
Actinidain was first identified in 1959 when A.C. Arcus looked into why jellies made with kiwifruit don’t solidify.[12] They went on to show that this phenomenon was caused by a proteolytic enzyme attacking gelatin.[12] This enzyme would go on to be named actinidin as it was identified in a fruit in the genus Actinidia.[12] While similar proteins have been found in other fruits, this cysteine protease is unique to the kiwifruit.[13][12] A thiol group was identified to be essential for enzyme activity, which is why it was grouped with enzymes like papain and bromelain.[14][15]
Function
While no clear function has been identified, the enzyme begins to accumulate in the fruit early on and is suspected to be important for fruit development.[16] Actinidain has been found to have a detrimental effect on the larvae of Spodoptera litura, however not enough research has been done into whether the enzyme can be used as a pesticide.[13] It may also be used as a storage protein.[17]
Sequence and structure
Actinidain has an enzyme classification number (EC) of 3.4.22.14. The 3 classifies it as a hydrolase.[18] It is further classified as acting on peptide bonds, also known as a peptidase (3.4). The .22 represents the cysteine endopeptidases and then the .14 is actinidain’s unique identifier within that group.[18] Actinidain is first produced in the kiwi when it is about half its size and then increases in both protease activity and enzyme production until the fruit is fully matured.[13] The enzyme is encoded by a large gene family and is expressed in most tissues of the kiwifruit plant, not just the fruit itself.[13]
Actinidain is similar to papain in size, shape, active site location and conformation, as well as in kinetic studies, which is especially interesting as they only share 48% amino acid similarity.[2][14] Electron density mapping shows similar α-helices and overall polypeptide folding.[2][14] While the electron density map indicates 218 amino acids, further sequencing work suggests 220 amino acids with the extra two being found at the C-terminus.[14][15] The active site includes cysteine and histidine residues that are conserved across several other proteins in the fruit peptidase family.[15] Electron density mapping indicates a double crossover with domain 1 being made up of AA 19-115 and 214-218 and domain II composing of AA 1-18 and 116-213,[14] with both the N-terminal and the C-terminal ends crossing over into both domains. Domain 1 has several α-helices whereas domain 2 is primarily made up of one anti-parallel β-sheet.[14] Actinidain comprises up to 50% of the kiwifruit’s soluble protein content at harvest.[19] Actinidain is active over a wide range of pH, including very acidic conditions,[20] with a pH optimum from 5-7.[21] At least ten different isoforms that all have the same molecular weight and cysteine protease activity as actinidain have been identified but they vary in isoelectric point from acidic (pI 3.9) to basic (pI 9.3).[19]
Human health impacts
Actinidain is able to function at low acidities (pH 1-2) that are found in the human GI tract and therefore is found to assist with protein digestion in the stomach and small intestine.[20][22] Actinidain enhances the human body’s ability to digest food, particularly when working together with pepsin and pancreatin, by hydrolyzing food proteins more efficiently than human digestive enzymes.[23] Further work is being done into the usefulness of kiwifruit as a digestive aid.
Actinidain is the major allergen in kiwifruit.[19][20] There does not appear to be any trend when looking at who is allergic to kiwi as it varies within age, geographical differences, and other characteristics clinicians use to track allergens, although the allergy often presents itself as mild symptoms in the mouth.[20] Actinidain provokes both IgG and IgE responses antibody responses, with the IgE binding activity being associated with the severe (anaphylaxis) responses.[19]
Potential applications
Actinidain is used as a high-quality meat tenderizer.[19] When marinating with pork, actinidain was found to tenderize it by affecting the myofibrils and the connective tissue, which are similar to the tissues that are broken down through mechanical tenderization.[24][25]
Studies have shown that actinidain might be a good alternative milk coagulant, replacing chymosin, a common coagulant used in cheese making.[26]
References
- ↑ "The specificity of actinidin and its relationship to the structure of the enzyme". Biochimica et Biophysica Acta (BBA) - Enzymology 616 (1): 30–34. November 1980. doi:10.1016/0005-2744(80)90260-0. PMID 7002215.
- ↑ 2.0 2.1 2.2 "Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain". Journal of Molecular Biology 182 (2): 317–329. March 1985. doi:10.1016/0022-2836(85)90348-1. PMID 3889350.
- ↑ "The thiol proteases: structure and mechanism". Active Sites of Enzymes. Biological Macromolecules and Assemblies. 3. New York: John Wiley and Sons. 1987. pp. 314–368. ISBN 978-0-471-85142-4. https://archive.org/details/biologicalmacrom0000unse/page/314.
- ↑ "Temperature-dependences of the kinetics of reactions of papain and actinidin with a series of reactivity probes differing in key molecular recognition features". The Biochemical Journal 396 (1): 17–21. May 2006. doi:10.1042/BJ20051501. PMID 16445383.
- ↑ "Diversity and relative levels of actinidin, kiwellin, and thaumatin-like allergens in 15 varieties of kiwifruit (Actinidia)". Journal of Agricultural and Food Chemistry 61 (3): 728–739. January 2013. doi:10.1021/jf304289f. PMID 23289429.
- ↑ "Kiwifruit cysteine protease actinidin compromises the intestinal barrier by disrupting tight junctions". Biochimica et Biophysica Acta (BBA) - General Subjects 1860 (3): 516–526. March 2016. doi:10.1016/j.bbagen.2015.12.005. PMID 26701113. https://cherry.chem.bg.ac.rs/handle/123456789/3559.
- ↑ "The effect of kiwifruit (Actinidia deliciosa) cysteine protease actinidin on the occludin tight junction network in T84 intestinal epithelial cells". Food and Chemical Toxicology 72: 61–68. October 2014. doi:10.1016/j.fct.2014.07.012. PMID 25042511.
- ↑ "Exogenous proteases for meat tenderization". Critical Reviews in Food Science and Nutrition 54 (8): 1012–1031. 2014. doi:10.1080/10408398.2011.623247. PMID 24499119.
- ↑ "Antibacterial effects of natural tenderizing enzymes on different strains of Escherichia coli O157:H7 and Listeria monocytogenes on beef". Meat Science 96 (4): 1494–1500. April 2014. doi:10.1016/j.meatsci.2013.12.010. PMID 24447905.
- ↑ "Production of novel dairy products using actinidin and high pressure as enzyme activity regulator". Innovative Food Science & Emerging Technologies 11 (1): 47–51. January 2010. doi:10.1016/j.ifset.2009.08.007.
- ↑ Ingredients in meat products properties, functionality and applications. New York: Springer. 2008. ISBN 978-0-387-71327-4. https://books.google.com/books?id=C-wrQaaXxj0C&q=actinidin+denaturation+temperature&pg=PA179.
- ↑ 12.0 12.1 12.2 12.3 "Proteolytic enzyme of Actinidia chinensis". Biochimica et Biophysica Acta 33 (1): 242–244. May 1959. doi:10.1016/0006-3002(59)90522-0. PMID 13651208.
- ↑ 13.0 13.1 13.2 13.3 "Effects of kiwifruit ( Actinidia deliciosa ) cysteine protease on growth and survival of Spodoptera litura larvae (Lepidoptera: Noctuidae) fed with control or transgenic avidin‐expressing tobacco" (in en). New Zealand Journal of Crop and Horticultural Science 33 (2): 99–105. June 2005. doi:10.1080/01140671.2005.9514337. ISSN 0114-0671. http://www.tandfonline.com/doi/abs/10.1080/01140671.2005.9514337.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 "Structure of actinidin: details of the polypeptide chain conformation and active site from an electron density map at 2-8 A resolution". Journal of Molecular Biology 115 (3): 263–277. September 1977. doi:10.1016/0022-2836(77)90154-1. PMID 592367.
- ↑ 15.0 15.1 15.2 "The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis". The Biochemical Journal 173 (1): 73–83. July 1978. doi:10.1042/bj1730073. PMID 687380.
- ↑ "Molecular analysis of actinidin, the cysteine proteinase of Actinidia chinensis". Plant Molecular Biology 10 (3): 193–202. May 1988. doi:10.1007/BF00027396. PMID 24277513.
- ↑ "Proteolytic activities of kiwifruit actinidin (Actinidia deliciosa cv. Hayward) on different fibrous and globular proteins: a comparative study of actinidin with papain". Applied Biochemistry and Biotechnology 172 (8): 4025–4037. April 2014. doi:10.1007/s12010-014-0812-7. PMID 24604128.
- ↑ 18.0 18.1 "Information on EC 3.4.22.14 - actinidain". https://www.brenda-enzymes.org/enzyme.php?ecno=3.4.22.14.
- ↑ 19.0 19.1 19.2 19.3 19.4 "Characterization of the allergenic potential of proteins: an assessment of the kiwifruit allergen actinidin". Journal of Applied Toxicology 34 (5): 489–497. May 2014. doi:10.1002/jat.2897. PMID 23754484.
- ↑ 20.0 20.1 20.2 20.3 "The nutritional and health attributes of kiwifruit: a review". European Journal of Nutrition 57 (8): 2659–2676. December 2018. doi:10.1007/s00394-018-1627-z. PMID 29470689.
- ↑ "Anionic proteinase from Actinidia chinensis. Preparation and properties of the crystalline enzyme". European Journal of Biochemistry 14 (2): 214–221. June 1970. doi:10.1111/j.1432-1033.1970.tb00280.x. PMID 5506167.
- ↑ "Actinidin enhances gastric protein digestion as assessed using an in vitro gastric digestion model". Journal of Agricultural and Food Chemistry 58 (8): 5068–5073. April 2010. doi:10.1021/jf903332a. PMID 20232890.
- ↑ "Actinidin enhances protein digestion in the small intestine as assessed using an in vitro digestion model". Journal of Agricultural and Food Chemistry 58 (8): 5074–5080. April 2010. doi:10.1021/jf903835g. PMID 20232891.
- ↑ "Injection of marinade with actinidin increases tenderness of porcine M. biceps femoris and affects myofibrils and connective tissue" (in en). Journal of the Science of Food and Agriculture 89 (9): 1607–1614. July 2009. doi:10.1002/jsfa.3633. ISSN 0022-5142. Bibcode: 2009JSFA...89.1607C.
- ↑ "Nature-derived ingredients as sustainable alternatives for tenderizing meat and meat products: an updated review" (in en). Food Biotechnology 37 (2): 136–165. 2023-04-03. doi:10.1080/08905436.2023.2201354. ISSN 0890-5436.
- ↑ "Actinidin: A Promising Milk Coagulating Enzyme" (in en). European Journal of Nutrition & Food Safety: 43–51. 2011-03-22. ISSN 2347-5641. https://journalejnfs.com/index.php/EJNFS/article/view/245.
External links
- The MEROPS online database for peptidases and their inhibitors: C01.007
- EC 3.4.22.14
- actinidain at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

