Biology:Gammaproteobacteria
| Gammaproteobacteria | |
|---|---|
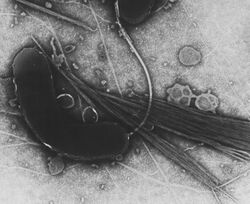
| |
| Vibrio cholerae | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria Garrity et al. 2005 |
| Orders | |
| |
| Synonyms | |
| |
Gammaproteobacteria is a class of bacteria in the phylum Pseudomonadota (synonym Proteobacteria). It contains about 250 genera, which makes it the most genus-rich taxon of the Prokaryotes.[1] Several medically, ecologically, and scientifically important groups of bacteria belong to this class. It is composed by all Gram-negative microbes and is the most phylogenetically and physiologically diverse class of Proteobacteria.[2]
These microorganisms can live in several terrestrial and marine environments, in which they play various important roles, including extreme environments such as hydrothermal vents. They generally have different shapes – rods, curved rods, cocci, spirilla, and filaments[3] and include free living bacteria, biofilm formers, commensals and symbionts,[4] some also have the distinctive trait of being bioluminescent.[5] Metabolisms found in the different genera are very different; there are both aerobic and anaerobic (obligate or facultative) species, chemolithoautotrophics, chemoorganotrophics, photoautotrophs and heterotrophs.[6]
Etymology
The element "gamma" (third letter of the Greek alphabet) indicates that this is Class III in Bergey's Manual of Systematic Bacteriology (Vol. II, page 1). Proteus refers to the Greek sea god who could change his shape. Bacteria (Greek βακτήριον; "rod" "little stick"), in terms of etymological history, refers to Bacillus (rod-shaped bacteria), but in this case is "useful in the interim while the phylogenetic data are being integrated into formal bacterial taxonomy."[7]
Phylogeny
Currently, many different classifications are based on different approaches, such as the National Center for Biotechnology Information, based on genomic, List of Prokaryotic names with Standing in Nomenclature , ARB-Silva Database[8] based on ribosomal RNA, or a multiprotein approach. It is still very difficult to resolve the phylogeny of this bacterial class.[9]
Here, it is reported a clade based on a set of 356 protein families for the class of Gammaproteobacteria.
| Phylogeny of Gammaproteobacteria | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phylogeny of Gammaproteobacteria after[10] Not all orders are monophyletic, consequently families or genera are shown for the Pseudomonadales, Oceanospirillales, and Alteromonadales. In the case of singleton orders, the genus is shown. (In bacterial taxonomy, orders have the suffix -ales, while families have -aceae.) |
A number of bacteria have been described as members of the Gammaproteobacteria, but have not yet been assigned an order or family. These include bacteria of the genera Alkalimonas, Gallaecimonas, Ignatzschineria, Litorivivens, Marinicella, Plasticicumulans, Pseudohongiella, Sedimenticola, Thiohalobacter, Thiohalorhabdus, Thiolapillus, and Wohlfahrtiimonas.[11]
Significance and applications
Gammaproteobacteria, especially the orders Alteromonadales and Vibrionales, are fundamental in marine and coastal ecosystems because they are the major groups involved in nutrient cycling[12] and despite their fame as pathogens, they find application in a huge number of fields, such as bioremediation and biosynthesis.
Gammaproteobacteria can be used as a microbial fuel cell (MFC)[13] element that applies their ability to dissimilate various metals.[14] The produced energy could be collected as one of the most environmentally friendly and sustainable energy production systems.[15] They are also used as biological methane filters.[16]
Phototrophic purple sulfur bacteria are used in wastewater treatment processes[17] and the ability of some Gammaproteobacteria (e.g. the genus Alcanivorax[18]) to bioremediate oil is becoming increasingly important to degrade crude oil after oil spills.[19] Some species from the family Chromatiaceae are notable because they may be involved in the production of vitamin B12.[20] Another application of some Gammaproteobacteria is their ability to synthesize Poly-b-hydroxyalkanoate (PHA)[21] which is a polymer that is used in the production of biodegradable plastics. Also many Gammaproteobacteria species are able to generate secondary metabolites with antibacterial properties.[22]
Ecology
Gammaproteobacteria are widely distributed and abundant in various ecosystems such as soil, freshwater lakes and rivers, oceans and salt lakes. For example, Gammaproteobacteria constitute about 6–20% (average of 14%) of bacterioplankton in different oceans;[23] plus, current researches have revealed their worldwide propagation in deep-sea and coastal sediments.[24] In seawater, Bacterial community composition could be shaped by miscellaneous environmental parameters, such as phosphorus, total organic carbon contents, salinity, and pH,[25] and the higher is the soil pH, the higher is the relative abundance of Alphaproteobacteria, Betaproteobacteria and Gammaproteobacteria.[26] The relative abundance of Betaproteobacteria and Gammaproteobacteria is also positively correlated to the dissolved organic carbon (DOC) concentration, which is a key environmental parameter shaping bacterial community composition.[27] Gammaproteobacteria are also key players in the dark carbon fixation in coastal sediments, which are the largest carbon sink on Earth and the majority of these bacteria have not been cultured yet.[28] The deep-sea hydrothermal system is one of the most extreme environments on Earth. Almost all vent-endemic animals are strongly associated with the primary production of the endo- and/or episymbiotic chemoautotrophic microorganisms.[29] Analyses of both the symbiotic and free-living microbial communities in the various deep-sea hydrothermal environments have revealed a predominance in biomass of members of the Gammaproteobacteria.[30]
Gammaproteobacteria have a wide diversity, metabolic versatility, and functional redundancy in the hydrothermal sediments, and they are responsible for the important organic carbon turnover and nitrogen and sulfur cycling processes.[31] Anoxic hydrothermal fluids contain several reduced compounds such as H2, CH4, and reduced metal ions in addition to H2S. It has been proposed that hydrogen sulfide-oxidizing and oxygen- reducing chemoautotrophs potentially sustain the primary production in these unique ecosystems.[32] In the last decades, it has been found that orders belonging to Gammaproteobacteria, like Pseudomonas, Moraxella, are able to degrade different types of plastics and these microbes might have a key role in plastic biodegradation.[33]
Metabolism
In the class of Gammaproteobacteria there is a wide diversity of metabolisms.[citation needed]
Some groups are nitrite-oxidizers[34] and ammonia oxidizers like the members of Nitrosococcus – with the exception of Nitrosococcus mobilis – and they are also obligate halophilic bacteria.[35]
Among Gammaproteobacteria there are chemoautotrophic sulfur-oxidizing groups, like Thiotrichales, which are found as microbial biofilm filamentous communities in the Tor Caldara shallow-water gas vent in the Tyrrhenian sea .[36] Moreover, thanks to 16S rRNA gene analysis, different sulfide oxidizers in the Gammaporteobacteria class have been detected, and the most important among them are Beggiatoa, Thioploca and Thiomargarita; besides, large amounts of hydrogen sulfide are produced by sulfate-reducing bacteria in organic-rich coastal sediments.[37]
Marine Gammaproteobacteria also include aerobic anoxygenic phototrophic bacteria (AAP) that use bacteriochlorophyll to support the electron transport chain. They are believed to be an essential community in the oceans and are also well spread all around.[38]
Another type of metabolism carried out by Gammaproteobacteria is the oxidation of Methane, carried out by the order Methylococcales. They metabolize methane as sole energy source and are very important in the global carbon cycle. They are found in any site where methane sources are, like gas reserves, soils, wastewaters.[39]
Purple sulfur bacteria are anoxygenic phototrophic iron‐oxidizers and they are part of the genus[40] Acidithiobacillus but, there are also two strains of Thiodictyon (Chromatiales order) -strain L7 and strain F4- and few species within the genus Thermomonas (order Lysobacter) that carry out the same metabolism.[41]
In this class, there are numerous genera of obligate and generalist hydrocarbonclastic bacteria. The obligate hydrocarbonoclastic bacteria (OHCB) share the ability to utilize hydrocarbons almost exclusively as a carbon source and until now they have been found only in the marine environment. The genera carrying out this metabolism are Alcanivorax, Oleiphilus, Oleispira, Thalassolitus, Cycloclasticus and Neptunomonas. Subsequently, additional species such as Polycyclovorans, Algiphilus of the order Xanthomonadales and Porticoccus hydrocarbonoclasticus of the order Cellvibrionales that were isolated from phytoplankton. Groups of aerobic “generalist” hydrocarbon degraders can utilize hydrocarbons and nonhydrocarbon substrates as source of carbon and energy and are members within the genera Acinetobacter, Colwellia, Glaciecola, Halomonas, Marinobacter, Marinomonas, Methylomonas, Pseudoalteromonas, Pseudomonas, Rhodanobacter, Shewanella, Stenotrophomonas, and Vibrio.[42]
The most frequent pathway to synthesize glucose among Gammaproteobacteria members is Calvin–Benson–Bassham (CBB) cycle but, a minority of species of this class may use the rTCA cycle.[43] Thioflavicoccus mobilis (free living gammaproteobacteria) and "Candidatus Endoriftia persephone" (symbiont of the giant tubeworm Riftia pachyptila), present the possibility of using the rTCA cycle in addition to the CBB cycle. It has been showed that some species of Gammaproteobacteria may express two different carbon fixation pathways simultaneously.[44]
Symbiosis
Symbiosis is a close and a long-term biological interaction between two different biological organisms. A large number of Gammaproteobacteria are able to join in a close endosymbiosis with various species. Evidence for this can be found in a wide variety of ecological niches: on the ground,[45][46] within plants,[47] or deep on the ocean floor.[48] On the land, it has been reported that Gammaproteobacteria species have been isolated from Robinia pseudoacacia[49] and other plants,[50][51] while in the deep sea a sulfur-oxidizing gammaproteobacteria was found in a hydrothermal vent chimney;[52] by entering into symbiotic relationships in deep sea areas, sulfur-oxidizing chemolithotrophic microbes receive additional organic hydrocarbons in hydrothermal ecosystems. Some Gammaproteobacteria are symbiotic with geothermic ocean vent-downwelling animals[53], and in addition, Gammaproteobacteria can have complex relationships with other species that live around thermal springs,[54] for example, with the shrimp Rimicaris exoculata living from hydrothermal vents on the Mid-Atlantic Ridge.
Regarding the endosymbionts, most of them lack many of their family characteristics due to significant genome reduction.[55][56]
Pathogens
Gammaproteobacteria comprise several medically and scientifically important groups of bacteria, such as the families Enterobacteriaceae, Vibrionaceae, and Pseudomonadaceae. A number of human pathogens belong to this class, including Yersinia pestis, Vibrio cholerae, Pseudomonas aeruginosa, Escherichia coli, and some species of Salmonella. The class also contains plant pathogens such as Xanthomonas axonopodis pv. citri (citrus canker), Pseudomonas syringae pv. actinidiae (kiwifruit Psa outbreak), and Xylella fastidiosa. In the marine environment, several species from this class can infect different marine organisms, such as species in the genus Vibrio which affect fish, shrimp, corals or oysters,[57] and species of Salmonella which affect grey seals (Halichoerus grypus).[58][59]
See also
References
- ↑ Garrity GM, Bell JA, Lilburn TG. (2005). Class III. Gammaproteobacteria class. nov. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM (eds) Bergey's Manual of Systematic Bacteriology 2nd edn, vol. 2 Springer: New York, p 1
- ↑ T. Gutierrez – 2019 - Institute of Mechanical, Process and Energy Engineering, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, UK
- ↑ "Dense populations of a giant sulfur bacterium in Namibian shelf sediments". Science 284 (5413): 493–5. April 1999. doi:10.1126/science.284.5413.493. PMID 10205058. Bibcode: 1999Sci...284..493S.
- ↑ "Phylogeny of gammaproteobacteria". Journal of Bacteriology 192 (9): 2305–14. May 2010. doi:10.1128/JB.01480-09. PMID 20207755.
- ↑ (in en) Marine Microbiology: Ecology & Applications. CRC Press. 2019-11-26. ISBN 978-0-429-59236-2. https://books.google.com/books?id=w6DLDwAAQBAJ&q=Colin%20Munn%20-%20Marine%20Microbiology%20Ecology%20and%20Applications%20(2011,%20Garland%20Science)&pg=PA80.
- ↑ "Proteobacteria | Microbiology". https://courses.lumenlearning.com/microbiology/chapter/proteobacteria/.
- ↑ Stackebrandt, E.; Murray, R. G. E.; Truper, H. G. (1988). "Proteobacteria classis nov., a Name for the Phylogenetic Taxon That Includes the "Purple Bacteria and Their Relatives"". International Journal of Systematic Bacteriology 38 (3): 321–325. doi:10.1099/00207713-38-3-321. ISSN 0020-7713.
- ↑ "Silva". https://www.arb-silva.de/.
- ↑ "Phylogeny of gammaproteobacteria". Journal of Bacteriology 192 (9): 2305–14. May 2010. doi:10.1128/JB.01480-09. PMID 20207755.
- ↑ "Phylogeny of gammaproteobacteria". Journal of Bacteriology 192 (9): 2305–14. May 2010. doi:10.1128/JB.01480-09. PMID 20207755.
- ↑ "Classification of domains and phyla - Hierarchical classification of prokaryotes (bacteria) - Gammaproteobacteria". List of Prokaryotic Names with Standing in Nomenclature. http://www.bacterio.net/-classifphyla.html#alkalimonas.
- ↑ "Ecology of type II secretion in marine gammaproteobacteria". Environmental Microbiology 10 (5): 1101–7. May 2008. doi:10.1111/j.1462-2920.2007.01545.x. PMID 18218035.
- ↑ "Miniaturizing microbial fuel cells". Trends in Biotechnology 29 (2): 62–9. February 2011. doi:10.1016/j.tibtech.2010.10.003. PMID 21075467.
- ↑ "Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms". Proceedings of the National Academy of Sciences of the United States of America 103 (30): 11358–63. July 2006. doi:10.1073/pnas.0604517103. PMID 16849424. Bibcode: 2006PNAS..10311358G.
- ↑ "Ecology and biotechnology of the genus Shewanella". Annual Review of Microbiology 61 (1): 237–58. September 2007. doi:10.1146/annurev.micro.61.080706.093257. PMID 18035608.
- ↑ The Prokaryotes: Gammaproteobacteria (4th ed.). Berlin Heidelberg: Springer-Verlag. 2014. pp. 434. ISBN 978-3-642-38921-4. https://www.springer.com/gp/book/9783642389214.
- ↑ "Treatment of industrial waste solutions and production of useful by-products using a photosynthetic bacterial method" (in en). Water Research 7 (8): 1219–1224. 1973-08-01. doi:10.1016/0043-1354(73)90075-4. ISSN 0043-1354. https://dx.doi.org/10.1016%2F0043-1354%2873%2990075-4.
- ↑ "Petroleum biodegradation in marine environments". Journal of Molecular Microbiology and Biotechnology 1 (1): 63–70. August 1999. PMID 10941786. https://pubmed.ncbi.nlm.nih.gov/10941786/#:~:text=Upon%20discharge%20into%20the%20sea,physical,%20chemical%20and%20biological%20modification.&text=In%20other%20words,%20the%20addition,the%20biodegradation%20of%20spilled%20oil.
- ↑ "Molecular detection of marine bacterial populations on beaches contaminated by the Nakhodka tanker oil-spill accident". Environmental Microbiology 3 (4): 246–55. April 2001. doi:10.1046/j.1462-2920.2001.00185.x. PMID 11359510. https://pubmed.ncbi.nlm.nih.gov/11359510/.
- ↑ "Extracellular metabolites from phototrophic bacteria as possible intermediates in the biosynthesis of vitamin B12". Fermentation Products (Pergamon): 247–252. ISBN 978-0-08-025385-5.
- ↑ "Taxonomic study of the genus Salinicola: transfer of Halomonas salaria and Chromohalobacter salarius to the genus Salinicola as Salinicola salarius comb. nov. and Salinicola halophilus nom. nov., respectively". International Journal of Systematic and Evolutionary Microbiology 60 (Pt 4): 963–971. April 2010. doi:10.1099/ijs.0.014480-0. PMID 19661506.
- ↑ "Marine bacteria associated with marine macroorganisms: The potential antimicrobial resources - AMiner". https://www.aminer.org/pub/5488f7f645ce471f9099557f.
- ↑ Broszat M, Nacke H, Blasi R, Siebe C, Huebner J, Daniel R, Grohmann E. 2014. Wastewater irrigation increases the abundance of potentially harmful Gammaproteobacteria in soils in Mezquital Valley, Mexico. Appl Environ Microbiol.
- ↑ "Diversity and Biogeography of Bathyal and Abyssal Seafloor Bacteria". PLOS ONE 11 (1): e0148016. 2016-01-27. doi:10.1371/journal.pone.0148016. PMID 26814838. Bibcode: 2016PLoSO..1148016B.
- ↑ "Microbial Diversity in the Deep Marine Sediments from the Qiongdongnan Basin in South China Sea". Geomicrobiology Journal 24 (6): 505–517. 2007-09-26. doi:10.1080/01490450701572473.
- ↑ "Soil bacterial and fungal communities across a pH gradient in an arable soil". The ISME Journal 4 (10): 1340–51. October 2010. doi:10.1038/ismej.2010.58. PMID 20445636.
- ↑ "Dissolved organic carbon influences microbial community composition and diversity in managed aquifer recharge systems". Applied and Environmental Microbiology 78 (19): 6819–28. October 2012. doi:10.1128/AEM.01223-12. PMID 22798375. Bibcode: 2012ApEnM..78.6819L.
- ↑ "Sedimentary organic matter preservation: an assessment and speculative synthesis.". Marine Chemistry 49 (2–3): 81–115. April 1995. doi:10.1016/0304-4203(95)00008-F.
- ↑ "Molecular ecology of hydrothermal vent microbial communities". Antonie van Leeuwenhoek 77 (2): 117–33. February 2000. doi:10.1023/a:1002463825025. PMID 10768471.
- ↑ "Chemosynthetic endosymbioses: adaptations to oxic-anoxic interfaces". Trends in Microbiology 13 (9): 439–48. September 2005. doi:10.1016/j.tim.2005.07.007. PMID 16054816.
- ↑ "Genomic resolution of linkages in carbon, nitrogen, and sulfur cycling among widespread estuary sediment bacteria". Microbiome 3: 14. 2015. doi:10.1186/s40168-015-0077-6. PMID 25922666.
- ↑ Jannasch,H.W.,andMottl,M.J. (1985). Geomicrobiologyofdeep- sea hydrothermalvents. Science 229, 717–725
- ↑ soixanteseize (2015-01-06). "Explore to understand, share to bring about change" (in en-US). https://oceans.taraexpeditions.org/en/m/science/news/bacterial-degradation-of-synthetic-plastics/.
- ↑ "Nitrite-Oxidizing Bacteria Community Composition and Diversity Are Influenced by Fertilizer Regimes, but Are Independent of the Soil Aggregate in Acidic Subtropical Red Soil" (in en). Frontiers in Microbiology 9: 885. 2018. doi:10.3389/fmicb.2018.00885. PMID 29867799.
- ↑ Cesar Mota, Jennifer Ridenoure, Jiayang Cheng, Francis L. de los Reyes, High levels of nitrifying bacteria in intermittently aerated reactors treating high ammonia wastewater. FEMS Microbiology Ecology, Volume 54, Issue 3, November 2005, pp. 391–400
- ↑ "Gammaproteobacteria During Colonization of a Shallow-Water Gas Vent" (in en). Frontiers in Microbiology 9: 2970. 2018. doi:10.3389/fmicb.2018.02970. PMID 30574130.
- ↑ Sabine Lenk, Julia Arnds, Katrice Zerjatke, Niculina Musat, Rudolf Amann and Marc Mußmann* Max Planck Institute for Marine Microbiology, Celsiusstraße 1, 28359 Bremen, Germany. Novel groups of Gammaproteobacteria catalyse sulfur oxidation and carbon fixation in a coastal, intertidal sediment. (2011)
- ↑ "Polyphyletic photosynthetic reaction centre genes in oligotrophic marine Gammaproteobacteria". Environmental Microbiology 9 (6): 1456–63. June 2007. doi:10.1111/j.1462-2920.2007.01264.x. PMID 17504483. https://sfamjournals.onlinelibrary.wiley.com/doi/abs/10.1111/j.1462-2920.2007.01264.x.
- ↑ "Methylococcales) Calls for the Reclassification of Members at the Genus and Species Levels" (in en). Frontiers in Microbiology 9: 3162. 2018. doi:10.3389/fmicb.2018.03162. PMID 30631317.
- ↑ "Microbial anaerobic Fe(II) oxidation - Ecology, mechanisms and environmental implications". Environmental Microbiology 20 (10): 3462–3483. October 2018. doi:10.1111/1462-2920.14328. PMID 30058270.
- ↑ Sabrina Hedrich,Michael Schlomann and D. Barrie Johnson. The iron-oxidizing proteobacteria. School of Biological Sciences, College of Natural Sciences, Bangor University, Deiniol Road, Bangor LL57 2UW, UK 2 Interdisciplinary Ecological Center, TU Bergakademie Freiberg, Leipziger Strasse 29, 09599 Freiberg, Germany. (2011)
- ↑ Terry J. McGenity, Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes, 2019 143-152; 181-189; 191-205.
- ↑ "Physiological proteomics of the uncultured endosymbiont of Riftia pachyptila". Science 315 (5809): 247–50. January 2007. doi:10.1126/science.1132913. PMID 17218528. Bibcode: 2007Sci...315..247M. https://www.science.org/doi/10.1126/science.1132913.
- ↑ "Genetic Evidence for Two Carbon Fixation Pathways (the Calvin-Benson-Bassham Cycle and the Reverse Tricarboxylic Acid Cycle) in Symbiotic and Free-Living Bacteria". mSphere 4 (1). January 2019. doi:10.1128/mSphere.00394-18. PMID 30602523.
- ↑ "Gammaproteobacteria as essential primary symbionts in the striped shield bug, Graphosoma Lineatum (Hemiptera: Pentatomidae)". Scientific Reports 6 (1): 33168. September 2016. doi:10.1038/srep33168. PMID 27609055. Bibcode: 2016NatSR...633168K.
- ↑ "Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae).". Applied Entomology and Zoology 47 (1): 1–8. February 2012. doi:10.1007/s13355-011-0081-7.
- ↑ "Profiling the Lolium perenne microbiome: from seed to seed.". Phytobiomes Journal 4 (3): 281–9. August 2020. doi:10.1094/PBIOMES-03-20-0026-R.
- ↑ "High‐contiguity genome assembly of the chemosynthetic gammaproteobacterial endosymbiont of the cold seep tubeworm Lamellibrachia barhami.". Molecular Ecology Resources 20 (5): 1432–44. September 2020. doi:10.1111/1755-0998.13220.
- ↑ "Nodulation in black locust by the Gammaproteobacteria Pseudomonas sp. and the Betaproteobacteria Burkholderia sp". Systematic and Applied Microbiology 33 (5): 269–74. August 2010. doi:10.1016/j.syapm.2010.04.005. PMID 20542651.
- ↑ "Ascorbic acid production in root, nodule and Enterobacter spp. (Gammaproteobacteria) isolated from root nodule of the legume Abrus precatorius L.". Biocatalysis and Agricultural Biotechnology 4 (2): 127–134. 2015-04-01. doi:10.1016/j.bcab.2014.11.006.
- ↑ "Gamma proteobacteria can nodulate legumes of the genus Hedysarum". Systematic and Applied Microbiology 27 (4): 462–8. August 2004. doi:10.1078/0723202041438527. PMID 15368852.
- ↑ "Physiological and genomic features of a novel sulfur-oxidizing gammaproteobacterium belonging to a previously uncultivated symbiotic lineage isolated from a hydrothermal vent". PLOS ONE 9 (8): e104959. 2014-08-18. doi:10.1371/journal.pone.0104959. PMID 25133584. Bibcode: 2014PLoSO...9j4959N.
- ↑ Holt JR (6 February 2013). "Description of the Phylum Gammaproteobacteria". http://comenius.susqu.edu/biol/202/eubacteria/proteobacteriae/gammaproteobacteria/default.htm.
- ↑ "Dual symbiosis of the vent shrimp Rimicaris exoculata with filamentous gamma- and epsilonproteobacteria at four Mid-Atlantic Ridge hydrothermal vent fields". Environmental Microbiology 12 (8): 2204–18. August 2010. doi:10.1111/j.1462-2920.2009.02129.x. PMID 21966914.
- ↑ "Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS". Nature 407 (6800): 81–6. September 2000. doi:10.1038/35024074. PMID 10993077. Bibcode: 2000Natur.407...81S.
- ↑ "Massive genomic decay in Serratia symbiotica, a recently evolved symbiont of aphids". Genome Biology and Evolution 3: 195–208. 2011. doi:10.1093/gbe/evr002. PMID 21266540.
- ↑ "Ecology of type II secretion in marine gammaproteobacteria". Environmental Microbiology 10 (5): 1101–7. May 2008. doi:10.1111/j.1462-2920.2007.01545.x. PMID 18218035.
- ↑ "Salmonella infection in grey seals (Halichoerus grypus), a marine mammal sentinel species: pathogenicity and molecular typing of Salmonella strains compared with human and livestock isolates". Environmental Microbiology 18 (3): 1078–87. March 2016. doi:10.1111/1462-2920.13219. PMID 26768299. http://doi.wiley.com/10.1111/1462-2920.13219.
- ↑ "Vibrio parahaemolyticus infections in the United States, 1973-1998". The Journal of Infectious Diseases 181 (5): 1661–6. May 2000. doi:10.1086/315459. PMID 10823766.
External links
- Gammaproteobacteria at the US National Library of Medicine Medical Subject Headings (MeSH)
Wikidata ☰ Q134668 entry
 |

