Biology:Biliverdin reductase
| biliverdin reductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 1.3.1.24 | ||||||||
| CAS number | 9074-10-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| biliverdin reductase A | |
|---|---|
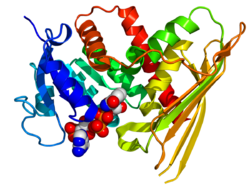 Crystallographic structure of human biliverdin reductase A based on the PDB: 2H63 coordinates. The enzyme is displayed as a rainbow colored cartoon (N-terminus = blue, C-terminus = red) while the NADP cofactor is displayed as space-filling model (carbon = white, oxygen = red, nitrogen = blue, phosphorus = orange). | |
| Identifiers | |
| Symbol | BLVRA |
| Alt. symbols | BLVR |
| NCBI gene | 644 |
| HGNC | 1062 |
| OMIM | 109750 |
| RefSeq | NM_000712 |
| UniProt | P53004 |
| Other data | |
| EC number | 1.3.1.24 |
| Locus | Chr. 7 p14-cen |
| biliverdin reductase B | |
|---|---|
| Identifiers | |
| Symbol | BLVRB |
| Alt. symbols | FLR |
| NCBI gene | 645 |
| HGNC | 1063 |
| OMIM | 600941 |
| RefSeq | NM_000713 |
| UniProt | P30043 |
| Other data | |
| EC number | 1.3.1.24 |
| Locus | Chr. 19 q13.1-13.2 |
| Biliverdin reductase, catalytic | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 crystal structure of a biliverdin reductase enzyme-cofactor complex | |||||||||
| Identifiers | |||||||||
| Symbol | Biliv-reduc_cat | ||||||||
| Pfam | PF09166 | ||||||||
| InterPro | IPR015249 | ||||||||
| SCOP2 | 1lc0 / SCOPe / SUPFAM | ||||||||
| |||||||||
Biliverdin reductase (BVR) is an enzyme (EC 1.3.1.24) found in all tissues under normal conditions, but especially in reticulo-macrophages of the liver and spleen. BVR facilitates the conversion of biliverdin to bilirubin via the reduction of a double-bond between the second and third pyrrole ring into a single-bond.
There are two isozymes, in humans, each encoded by its own gene, biliverdin reductase A (BLVRA) and biliverdin reductase B (BLVRB).
Mechanism of catalysis
BVR acts on biliverdin by reducing its double-bond between the pyrrole rings into a single-bond.[1] It accomplishes this using NADPH + H+ as an electron donor, forming bilirubin and NADP+ as products.
BVR catalyzes this reaction through an overlapping binding site including Lys18, Lys22, Lys179, Arg183, and Arg185 as key residues.[2] This binding site attaches to biliverdin, and causes its dissociation from heme oxygenase (HO) (which catalyzes reaction of ferric heme --> biliverdin), causing the subsequent reduction to bilirubin.[3]
Structure
BVR is composed of two closely packed domains, between 247-415 amino acids long and containing a Rossmann fold.[4] BVR has also been determined to be a zinc-binding protein with each enzyme protein having one strong-binding zinc atom.[5][6]
The C-terminal half of BVR contains the catalytic domain, which adopts a structure containing a six-stranded beta-sheet that is flanked on one face by several alpha-helices. This domain contains the catalytic active site, which reduces the gamma-methene bridge of the open tetrapyrrole, biliverdin IX alpha, to bilirubin with the concomitant oxidation of a NADH or NADPH cofactor.[7]
Function
BVR works with the biliverdin/bilirubin redox cycle. It converts biliverdin to bilirubin (a strong antioxidant), which is then converted back into biliverdin through the actions of reactive oxygen species (ROS). This cycle allows for the neutralization of ROS, and the reuse of biliverdin products. Biliverdin also is replenished in the cycle with its formation from heme units through heme oxygenase (HO) localized from the endoplasmic reticulum.[8]
Bilirubin, being one of the last products of heme degradation in the liver, is further processed and excreted in bile after conjugation with glucuronic acid.[9] In this way, BVR is essential in many mammals for the disposal of heme catabolites – especially in the fetus where the placental membranes are bilirubin-permeable but not biliverdin-permeable - aiding in the removal of potentially toxic protein build-up.[10]
BVR has also more recently been recognized as a regulator of glucose metabolism and in cell growth and apoptosis control, due to its dual-specificity kinase character.[11] This control over glucose metabolism indicates that BVR may play a role in pathogenesis of multiple metabolic diseases - the notable one being diabetes, by control of the upstream activator of insulin growth factor-1 (IGF-1) and mitogen-activated protein kinase (MAPK) signaling pathway.[12]
Disease relevance
BVR acts as a means to regenerate bilirubin in a repeating redox cycle without significantly modifying the concentration of available bilirubin. With these levels maintained, it appears that BVR represents a new strategy for the treatment of multiple sclerosis and other types of oxidative stress-mediated diseases.[13] The mechanism is due to the amplification of the potent antioxidant actions of bilirubin, as this can ameliorate free radical-mediated diseases.[14]
Studies have shown that the BVR redox cycle is essential in providing physiological cytoprotection. Genetic knock-outs and reduced BVR levels have demonstrated increased formation of ROS, and results in augmented cell death. Cells that experienced a 90% reduction in BVR experienced three times normal ROS levels.[15] Through this protective and amplifying cycle, BVR allows low concentrations of bilirubin to overcome 10,000-fold higher concentrations of ROS.[16]
References
- ↑ "The reaction mechanism of bovine kidney biliverdin reductase". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 957 (2): 237–42. Nov 1988. doi:10.1016/0167-4838(88)90278-6. PMID 3191141.
- ↑ "The binding sites on human heme oxygenase-1 for cytochrome p450 reductase and biliverdin reductase". The Journal of Biological Chemistry 278 (22): 20069–76. May 2003. doi:10.1074/jbc.M300989200. PMID 12626517.
- ↑ "Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress". The Journal of Biological Chemistry 277 (11): 9226–32. Mar 2002. doi:10.1074/jbc.M108239200. PMID 11773068.
- ↑ "The nicotinamide dinucleotide binding motif: a comparison of nucleotide binding proteins". FASEB Journal 10 (11): 1257–69. Sep 1996. doi:10.1096/fasebj.10.11.8836039. PMID 8836039.
- ↑ "Human biliverdin IXalpha reductase is a zinc-metalloprotein. Characterization of purified and Escherichia coli expressed enzymes". European Journal of Biochemistry 235 (1–2): 372–81. Jan 1996. doi:10.1111/j.1432-1033.1996.00372.x. PMID 8631357.
- ↑ PDB: 1GCU; "Crystal structure of rat biliverdin reductase". Nature Structural Biology 8 (3): 221–5. Mar 2001. doi:10.1038/84955. PMID 11224565.
- ↑ "Crystal structure of a biliverdin IXalpha reductase enzyme-cofactor complex". Journal of Molecular Biology 319 (5): 1199–210. Jun 2002. doi:10.1016/S0022-2836(02)00383-2. PMID 12079357.
- ↑ "Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1". The Journal of Biological Chemistry 279 (19): 19916–23. May 2004. doi:10.1074/jbc.M314251200. PMID 14988408.
- ↑ "Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man". The Journal of Biological Chemistry 269 (27): 17960–4. Jul 1994. doi:10.1016/S0021-9258(17)32403-1. PMID 8027054.
- ↑ "Reduction of biliverdin and placental transfer of bilirubin and biliverdin in the pregnant guinea pig". The Biochemical Journal 194 (1): 273–82. Jan 1981. doi:10.1042/bj1940273. PMID 7305981.
- ↑ "Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance". Pharmacological Reports 60 (1): 38–48. January–February 2008. PMID 18276984.
- ↑ "Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin". Trends in Pharmacological Sciences 30 (3): 129–37. Mar 2009. doi:10.1016/j.tips.2008.12.003. PMID 19217170.
- ↑ "Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase". The Journal of Biological Chemistry 284 (43): 29251–9. Oct 2009. doi:10.1074/jbc.M109.037119. PMID 19690164.
- ↑ "Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis". Journal of Immunology 181 (3): 1887–97. Aug 2008. doi:10.4049/jimmunol.181.3.1887. PMID 18641326.
- ↑ "Biliverdin reductase: a major physiologic cytoprotectant". Proceedings of the National Academy of Sciences of the United States of America 99 (25): 16093–8. Dec 2002. doi:10.1073/pnas.252626999. PMID 12456881. Bibcode: 2002PNAS...9916093B.
- ↑ "Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle". Pediatrics 113 (6): 1776–82. Jun 2004. doi:10.1542/peds.113.6.1776. PMID 15173506.
External links
- biliverdin+reductase at the US National Library of Medicine Medical Subject Headings (MeSH)
 |


