Biology:Australian bat lyssavirus
| Australian bat lyssavirus | |
|---|---|
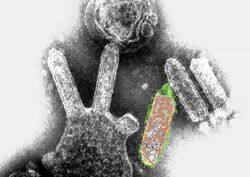
| |
| Colored transmission electron micrograph of Australian bat lyssavirus. The bullet-like objects are the virions, and some of them are budding off from a cell. | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Rhabdoviridae |
| Genus: | Lyssavirus |
| Species: | Australian bat lyssavirus
|
Australian bat lyssavirus (ABLV), originally named Pteropid lyssavirus (PLV), is a enzootic virus closely related to the rabies virus. It was first identified in a 5-month-old juvenile black flying fox (Pteropus alecto) collected near Ballina in northern New South Wales, Australia , in January 1995 during a national surveillance program for the recently identified Hendra virus.[1] ABLV is the seventh member of the genus Lyssavirus (which includes Rabies virus) and the only Lyssavirus member present in Australia. ABLV has been categorized to the Phylogroup I of the Lyssaviruses.[2]
Virology
Molecular structure
The Australian bat lyssavirus (ABVL) shares many structural characteristics with the other Lyssaviruses, despite being genetically and serologically distinct from the others.[2] Visually, ABLV is a bullet shaped virus. Molecularly, ABVL is an enveloped, negative-sense, single-stranded RNA virus. The (-)ssRNA genome is relatively small, containing 12kilobases of genetic material and encoding five viral proteins. The five viral proteins, their symbol, and their functional roles are:
| Symbol | Name | Structure | Function |
|---|---|---|---|
| N | Nucleoprotein | Interacts with genome, P, and L to form the ribonucleoprotein complex | Encapsulates the RNA genome, making it available for transcription and genome replication |
| P | Phosphoprotein | Cofactor for L | Regulatory functions for viral replication
Helps virus evade host immune system - suppressing host immunity |
| M | Matrix | Physically connecting the viral capsid to the host derived membrane
Interacts with P and L |
Aids in viral assembly, coordinates translation and genome replication, and directs budding |
| G | Glycoprotein | Trimeric surface protein spikes, associated with M and extends through the host cell derived membrane envelope | Utilized for host cell entry with receptor-mediated endocytosis |
| L | RNA dependent RNA polymerase | Enzyme for transcription (produce mRNA from genome) and viral genome replication |
Viral entry mechanism and intracellular trafficking
ABLV has a similar entry mechanism to other rabies viruses, utilizing receptor-mediated endocytosis by the host cells. The glycoprotein (G) is a trimeric spike protein that extends through the virus's envelope and can interact with surface receptors of host cells. While the specific receptors remain mostly unknown at this time, it is thought that ABLV enters the nervous system of host through the neuromuscular junction of the peripheral nervous system. Additionally, it is believed that the spike protein either binds to a highly specific host receptor or uses a co-receptor in lipid rafts.[3] Some of the proposed receptors include nicotinic acetylcholine receptor (nAchR), p75 neurotrophin receptor (p75NTR), and neuronal cell adhesion molecule (NCAM).[5]
After attaching to the host cell surface, ABLV uses a clathrin-dynamin-dependent pathway to invaginate the host membrane and pinch off a vesicle. Actin polymerization at the site of invagination is also required for successful viral entry. The virus is endocytosed fully, unenveloped. The vesicle fuses with a lysosome, causing the pH within the infected vesicle to drop. The lowering of pH in the early-endosome causes a conformational change in the spike protein G. This allows the viral envelope to fuse with the endosome, releasing the nucleocapsid into the cytoplasm of the host cell.[3]
The virus utilizes the host's intracellular trafficking mechanisms for its own transportation. The microtubule network and motors, kinesin and dynein, transport cellular products in anterograde and retrograde transport respectively. After the nucleocapsid is released from the early endosome, it associates with dynein motors to travels through the axon of the infected neuron towards the cell body. The use of the microtubule network and motors is to overall move the virus from the peripheral nervous system to the central nervous system, which is a more favorable environment for the virus and where neurological symptoms occur.[6]
Prevalence
Host reservoir and susceptible species
Bats, both flying foxes and insectivorous bats, are the only known host reservoir for ABLV. The known species of bat reservoirs are the Black Flying Fox (Pteropus alecto), the Grey-headed Flying Fox (P. poliocephalus), the Spectacled Flying Fox (P. conscpicullatus), the Little Red Flying Fox (P. scapulatus), and the Yellow-bellied Sheathtail Bat (Saccolaimus flaviventris).[5] These species are distributed throughout the Australian continent, and ABLV has only been serologically and phylogenetically in Australia.[7]:251 It is estimated that less than 1% of healthy bats are ABLV carriers. As for sick or injured bats, it is estimated that 5-10% have been infected, detected with fluorescent antibody testing.[5]
As for other species that are susceptible to ABLV infection, human and horse cases have been reported since 1996 and 2013, respectively. No other terrestrial animals have been reported to be infected with ABLV, despite known exposure to infected bats. However, recent studies have found that the ABLV receptor for host cell entry is conserved amongst a variety of mammals, including but not limited to small rodents, monkeys, and rabbits.[5]
Geographical distribution
As of now, ABLV has only been isolated and reported in Australia. The distribution of ABLV across the Australian continent is based on the ecological distribution of the bat reservoirs. From the four flying fox species identified as host reservoirs, ABLV is present in areas of Western Australia, North Territory, Queensland, New South Wales, and Victoria. From the Yellow-bellied Sheathtail Bat, ABLV is present throughout mainland Australia.[5]
Human Health
Zoonotic incidences
Three cases of ABLV in humans have been confirmed, all of which were fatal after an average of 20 days from the first presentation of symptoms. The incubation time of the virus varied widely for each case and cannot be explained at this time. The length of incubation is unusual as classical rabies has typical incubation periods of less than 90 days.
The first case occurred in November 1996, when an animal caregiver in Rockhampton sustained several scratches from fruit bats she was working with. She reported to the hospital four to five weeks later for shoulder pain, dizziness, vomiting, headache, fever, and chills. While hospitalized, her condition rapidly deteriorated, with slurred speech, diplopia (double-vision), dysphagia (difficulty swallowing), and progressive weakness in her limbs. From cerebrospinal fluid samples, no organisms were found with microscopy or culturing, despite elevated white blood cell levels. She was treated with several broad spectrum antibiotics with no improvement. An electroencephalogram was performed and found diffuse encephalitis. She eventually fell into a depressed conscious state, with a single incidence of extreme agitation. By her 11th day of hospitalization, she was fully ventilation dependent, nonresponsive, and hyperthermic. She died 20 days after her initial admittance. ABLV was identified from brain tissue by polymerase chain reaction and immunohistochemistry.[5][8][9]
The second case began in August 1996, a middle-aged woman in Queensland was bitten on the finger by a flying fox while attempting to remove it from a child on whom it had landed. Six months later, following heightened public attention from the first ABLV death, she consulted a general practitioner regarding testing for the virus. Post-exposure prophylaxis was advised, but for an unknown reason she declined the treatment. After a 27-month incubation, a rabies-like illness developed in November of 1998. She came in with symptoms of a fever, vomiting, pain in her shoulder, dysphagia, and muscle spasms. Her condition worsened after hospital admission, as her dysphagia increased, her muscle spasms became more pronounced and frequent, and she became increasing agitated. She became ventilation dependent and unable to communicate due to full paralysis. On the day the woman was hospitalized, cerebrospinal fluid, serum, and saliva were submitted for testing.[10] On the fourth day of her hospital admission, these tests were returned with results of probable ABLV infection. ABLV infection was confirmed by PCR on the 8th day of hospitalization. She died 19 days after the onset of illness. Postmortem tests were all strongly positive for ABLV.[5][10]
The third, and most recent, case occurred in December of 2012, when an eight-year-old boy was scratched by a bat in north Queensland. He became ill eight weeks later, showing symptoms including fever, anorexia, and abdominal pain. His condition worsened through his hospitalization, with abnormal and aggressive bouts between normal behavior and intense muscle spasms. He repeatedly needed to be extubated and sedated due to his spasms. The hospital performed several tests through his stay, including sending cerebrospinal fluid and blood samples off for testing, taking computed tomography images of his chest and abdomen, and performing neuroimaging (MRIs, electroencephalography's). Initially, tests for the ABLV antigens were negative, but repeated testing 12 days into his hospitalization provided positive results. The child died 28 days after the onset of symptoms on February 22, 2013.[5][11][12]
Pathology and pathogenesis of ABLV in humans
ABLV (and the other Lyssaviruses) present similarly to the traditional encephalitic rabies virus (RABV) in humans. The symptoms first are flu-like with fevers, headaches, dizziness, and an overall lethargy. In addition, there could be itching or pain at the site of the bat-bite. The symptoms progress with outbursts of agitation, confusion, slurred speech, muscle spasms, dysphagia, and weakness in all limbs.[13] Different from rabies virus, hydrophobia was not described in any of the three ABLV human cases. However, it should not be discounted as a possible symptom for ABLV. Once the disease has shown symptoms, it is almost always fatal. For ABLV specifically, there have been no survivors.
The pathogenesis of ABLV is still widely unknown and still being studied. It is hypothesized that the virus travels intracellularly through peripheral nervous system neurons to the central nervous system and brain. As mentioned prior, the virus uses the microtubular motor system of the host to travel intracellularly.
A difference between rabies virus and ABLV is the length of incubation time after initial infection from bat exposure. In traditional encephalitic rabies, the incubation is generally between 20 and 90 days.[14] For the three reported ABLV cases in humans, the incubation period ranged from a few weeks to almost two years.
Current Treatments for ABLV
Due to its difficulty in diagnosing, low number of reported cases, and relative novelty as an endemic virus, there are no successful treatment plans once the symptoms have begun. As previously stated, all intervention methods used on the three human cases of ABLV were not curative and all three cases resulted in fatality.
However, it is highly recommended by physicians to receive the RABV post-exposure prophylaxis (PEP) protocol immediately after potential lyssavirus exposure (i.e.. exposure/interaction with bats). Additionally, the incident should be reported to the relevant public health unit. Currently, the PEP protocol involves thoroughly cleaning the wound and surrounding tissue, administration of the rabies vaccine, and administration of rabies immunoglobulin (RIG).[15][16] Currently, there are two effective variations of RIGs used in PEP protocol, human RIG (HRIG) and equine RIG (ERIG). Drawbacks for HRIG are that there are limited supplies and the cost of production is high. HRIG is overall inaccessible for the general population. Drawbacks for ERIG are potential immunogenicity, which is when the immune system recognizes the RIG as foreign and causes an immune reaction.[17] In a recent study, performed in 2021 by Weir, Coggins, et.al., a new treatment method was proposed that used human monoclonal antibodies over RIGs. They identified two (A6 and F11) that recognized the G protein of lyssaviruses in phylogroup I (including ABLV) and completely neutralized the virus. They also proposed it as a potential diagnostic tool, in which there are only limited methods with PCR and are late into the symptomatic phase to positively identify ABLV.[15]
Prevention
Rabies vaccine and immunoglobulin are effective in prophylactic and therapeutic protection from ABLV infection. Since the emergence of the virus, rabies vaccine is administered to individuals with a heightened risk of exposure, and vaccine and immunoglobulin are provided for post-exposure treatment.
The public health units in Australia advise that the population avoid and limit their interactions and physical contact with bats as much as possible. ABLV is one of four zoonotic viruses discovered in pteropid bats since 1994, the others being Hendra virus, Nipah virus, and Menangle virus. Of these, ABLV is the only virus known to be transmissible to humans directly from bats without an intermediate host. Thus education and awareness in the general population is a must. If a bat is found and/or appears injured, one should avoid contact with it and instead call local pest and animal control to appropriately remove the bat.[18][19]
Non-human health
ABLV has also been reported to have the ability to transfer to horses. Currently, this is the only other known susceptible species.
ABLV was confirmed in two horses on Queensland's Darling Downs in May 2013. Both horses were euthanized when their condition deteriorated despite treatment and the attending veterinarian performed a post mortem examination obtaining samples that allowed for the laboratory diagnosis. The property was then quarantined. Three dogs and the four horses in closest contact received post exposure prophylaxis, as did all nine in-contact people. The virus was isolated and identified as the insectivorous bat strain. These cases have prompted reconsideration of the potential spillover of ABLV into domestic animal species. Veterinarians are urged to consider ABLV as a differential diagnosis in cases of progressive generalized neurological disease.[5][20]
See also
- European bat lyssavirus 1
References
- ↑ * "Australian bat lyssavirus infection in three fruit bats from north Queensland". Communicable Diseases Intelligence 21 (9): 117–120. May 1997. PMID 9145563. http://www.health.gov.au/internet/main/publishing.nsf/content/cda-cdi2109-pdf-cnt.htm/$FILE/cdi2109a.pdf. Retrieved 26 May 2011.
- ↑ 2.0 2.1 "Isolation and Characterization of Cross-Reactive Human Monoclonal Antibodies That Potently Neutralize Australian Bat Lyssavirus Variants and Other Phylogroup 1 Lyssaviruses". Viruses 13 (3): 391. March 2021. doi:10.3390/v13030391. PMID 33804519.
- ↑ 3.0 3.1 3.2 "Recent observations on Australian bat lyssavirus tropism and viral entry". Viruses 6 (2): 909–926. February 2014. doi:10.3390/v6020909. PMID 24556791.
- ↑ "Rabies Infection: An Overview of Lyssavirus-Host Protein Interactions". Iranian Biomedical Journal 25 (4): 226–242. July 2021. doi:10.52547/ibj.25.4.226. PMID 34217155.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "Recent observations on Australian bat lyssavirus tropism and viral entry". Viruses 6 (2): 909–926. February 2014. doi:10.3390/v6020909. PMID 24556791.
- ↑ "Early events in rabies virus infection-Attachment, entry, and intracellular trafficking". Virus Research 263: 217–225. April 2019. doi:10.1016/j.virusres.2019.02.006. PMID 30772332.
- ↑ * "Bats and lyssaviruses". Research Advances in Rabies. Advances in Virus Research. 79. Elsevier. 2011. pp. 239–289. doi:10.1016/B978-0-12-387040-7.00012-3. ISBN 978-0-12-387040-7.
- ↑ "A human case of encephalitis due to a lyssavirus recently identified in fruit bats.". Commun. Dis. Intellig. 20: 504. 1996. https://www1.health.gov.au/internet/main/publishing.nsf/Content/1996%20issues-1/$FILE/cdi2024.pdf.
- ↑ "Non-rabies Lyssavirus human encephalitis from fruit bats: Australian bat Lyssavirus (pteropid Lyssavirus) infection". Neuropathology and Applied Neurobiology 24 (4): 331–335. August 1998. doi:10.1046/j.1365-2990.1998.00129.x. PMID 9775399.
- ↑ 10.0 10.1 "Australian bat lyssavirus infection: a second human case, with a long incubation period". The Medical Journal of Australia 172 (12): 597–599. June 2000. doi:10.5694/j.1326-5377.2000.tb124126.x. PMID 10914106. http://www.mja.com.au/public/issues/172_12_190600/hanna/hanna.html. Retrieved 26 July 2008.
- ↑ "Child dies of bat virus in Brisbane hospital". Adelaidenow. http://www.adelaidenow.com.au/news/breaking-news/boy-dies-after-contracting-bat-virus/story-e6frea7l-1226583924440.[yes|permanent dead link|dead link}}]
- ↑ "Australian Bat Lyssavirus in a child: the first reported case". Pediatrics 133 (4): e1063–e1067. April 2014. doi:10.1542/peds.2013-1782. PMID 24590754.
- ↑ "What are the signs and symptoms of rabies? | Symptoms" (in en-us). 2022-02-16. https://www.cdc.gov/rabies/symptoms/index.html.
- ↑ "LA County Department of Public Health". http://publichealth.lacounty.gov/vet/rabiesmanual/clinical.htm#:~:text=The%20incubation%20period%20in%20humans,the%20amount%20of%20virus%20introduced..
- ↑ 15.0 15.1 "Isolation and Characterization of Cross-Reactive Human Monoclonal Antibodies That Potently Neutralize Australian Bat Lyssavirus Variants and Other Phylogroup 1 Lyssaviruses". Viruses 13 (3): 391. March 2021. doi:10.3390/v13030391. PMID 33804519.
- ↑ "Australian bat lyssavirus: implications for public health". The Medical Journal of Australia 201 (11): 647–649. December 2014. doi:10.5694/mja13.00261. PMID 25495308.
- ↑ "Immunogenicity - an overview | ScienceDirect Topics". https://www.sciencedirect.com/topics/medicine-and-dentistry/immunogenicity.
- ↑ "Don't handle bats, Vic health chief warns". The Sydney Morning Herald. 25 May 2011. http://news.smh.com.au/breaking-news-national/dont-handle-bats-vic-health-chief-warns-20110525-1f3d8.html.
- ↑ "It's batty not to cleanse this scourge". 2011-07-17. http://www.heraldsun.com.au/opinion/its-batty-not-to-cleanse-this-scourge/story-e6frfhqf-1226096070582.
- ↑ "Australian bat lyssavirus infection in two horses". Veterinary Microbiology 173 (3–4): 224–231. October 2014. doi:10.1016/j.vetmic.2014.07.029. PMID 25195190. http://era.daf.qld.gov.au/id/eprint/4578/.
Additional reading
- "Non-rabies Lyssavirus human encephalitis from fruit bats: Australian bat Lyssavirus (pteropid Lyssavirus) infection". Neuropathology and Applied Neurobiology 24 (4): 331–335. August 1998. doi:10.1046/j.1365-2990.1998.00129.x. PMID 9775399.
- "Encephalitis caused by a Lyssavirus in fruit bats in Australia". Emerging Infectious Diseases 2 (4): 327–331. 1996. doi:10.3201/eid0204.960408. PMID 8969249.
- "Clinical review of two fatal equine cases of infection with the insectivorous bat strain of Australian bat lyssavirus". Australian Veterinary Journal 92 (9): 324–332. September 2014. doi:10.1111/avj.12227. PMID 25156050.
External links
- "Australian Bat Lyssavirus". Queensland Health. http://access.health.qld.gov.au/hid/InfectionsandParasites/ViralInfections/australianBatLyssavirus_fs.asp.
Wikidata ☰ Q3269802 entry
 |