Chemistry:Tiopronin
| |
| Names | |
|---|---|
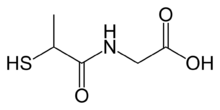
| IUPAC name
N-(2-Sulfanylpropanoyl)glycine
| |
| Systematic IUPAC name
(2-Sulfanylpropanamido)acetic acid | |
| Other names
2-mercaptopropionylglycine
Acadione | |
| Identifiers | |
3D model (JSmol)
|
|
| 1859822 | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | Tiopronin |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C5H9NO3S | |
| Molar mass | 163.19 g·mol−1 |
| Appearance | White, opaque crystals |
| Melting point | 93 to 98 °C (199 to 208 °F; 366 to 371 K) |
| log P | −0.674 |
| Acidity (pKa) | 3.356 |
| Basicity (pKb) | 10.641 |
| Pharmacology | |
| 1=ATC code }} | G04BX16 (WHO) QG04BX16 (WHO) |
| By mouth | |
| Legal status | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | WARNING |
| H302 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1,300 mg kg−1 (oral, rat) |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Clinical data | |
|---|---|
| Trade names | Thiola |
| License data | |
| Identifiers | |
| DrugBank | |
| ChEBI | |
Tiopronin, sold under the brand name Thiola, is a medication used to control the rate of cystine precipitation and excretion in the disease cystinuria.[3][4]
It is available as a generic medication.[5][6]
Medical uses
Tiopronin is indicated, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation in people 20 kilograms (44 lb) and greater with severe homozygous cystinuria, who are not responsive to these measures alone.[1][2]
Side effects
Tiopronin may present a variety of side effects, which are broadly similar to those of D-penicillamine and other compounds containing active sulfhydryl groups.[7] Its pharmacokinetics have been studied.[8]
Pharmacology
Mechanism of action
Tiopronin works by reacting with urinary cysteine to form a more soluble, disulfide linked, tiopronin-cysteine complex.[8]
Society and culture
In the U.S., the drug was marketed by Mission Pharmacal at $1.50 per pill, but in 2014 the rights were bought by Retrophin, owned by Martin Shkreli, and the price increased to $30 per pill for a 100 mg capsule.[9][10]
In 2016 Imprimis Pharmaceuticals introduced a lower cost version marketed as a compounded drug.[11]
Research
It may also be used for Wilson's disease (an overload of copper in the body), and has also been investigated for the treatment of arthritis,[12][13] though tiopronin is not an anti-inflammatory.[citation needed]
Tiopronin is also sometimes used as a stabilizing agent for metal nanoparticles. The thiol group binds to the nanoparticles, preventing coagulation.[14]
References
- ↑ 1.0 1.1 "Thiola- tiopronin tablet, sugar coated". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=494a714e-923c-cd57-df6c-12886afb265a.
- ↑ 2.0 2.1 "Thiola EC- tiopronin tablet, delayed release". 15 March 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=20298a52-a194-a161-8632-d84b6f26e23c.
- ↑ "Clinical course and cystine stone formation during tiopronin treatment". Urological Research 23 (2): 111–7. 1995. doi:10.1007/BF00307941. PMID 7676533.
- ↑ "The pathogenesis and treatment of kidney stones". The New England Journal of Medicine 327 (16): 1141–52. October 1992. doi:10.1056/NEJM199210153271607. PMID 1528210.
- ↑ "Competitive Generic Therapy Approvals". 29 June 2023. https://www.fda.gov/drugs/generic-drugs/competitive-generic-therapy-approvals.
- ↑ "First Generic Drug Approvals 2023". 30 May 2023. https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals.
- ↑ "Adverse effects profile of sulfhydryl compounds in man". The American Journal of Medicine 80 (3): 471–6. March 1986. doi:10.1016/0002-9343(86)90722-9. PMID 2937293.
- ↑ 8.0 8.1 "Pharmacokinetics of oral tiopronin". European Journal of Clinical Pharmacology 45 (1): 79–84. August 1993. doi:10.1007/BF00315354. PMID 8405034.
- ↑ "The Most Unconscionable Drug Price Hike I Have Yet Seen". In the Pipeline. 11 September 2014. https://www.science.org/content/blog-post/most-unconscionable-drug-price-hike-i-have-yet-seen.
- ↑ "Why would Martin Shkreli hike an old drug price by 5000%? Only a 'moron' would ask". FierceBiotech. September 20, 2015. http://www.fiercebiotech.com/story/why-would-martin-shkreli-hike-old-drug-price-5000-only-moron-would-ask/2015-09-20.
- ↑ "Imprimis shuts down Texas plant, axes 8% of jobs". BioPharma Dive. September 29, 2016. https://www.biopharmadive.com/news/imprimis-shuts-down-texas-plant-axes-8-of-jobs/427296/.
- ↑ "[Tolerability and therapeutic maintenance of tiopronin, new basic treatment of rheumatoid arthritis. Apropos of long-term follow-up of 268 cases]". Revue du Rhumatisme et des Maladies Osteo-Articulaires 56 (5 Pt 2): 38–42. April 1989. PMID 2740804.
- ↑ "Controlled multicenter trial of tiopronin and d-penicillamine for rheumatoid arthritis". Arthritis and Rheumatism 25 (8): 923–9. August 1982. doi:10.1002/art.1780250803. PMID 7115451.
- ↑ "Toward greener nanosynthesis". Chemical Reviews 107 (6): 2228–69. June 2007. doi:10.1021/cr050943k. PMID 17564480.
External links
- "Tiopronin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tiopronin.
 |

