Chemistry:Pentanal
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentanal | |
| Other names
Pentanaldehyde
Valeraldehyde Valeric aldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.134 g·mol−1 |
| Appearance | Clear liquid |
| Odor | Strong, acrid, pungent |
| Density | 0.8095 at 20 °C |
| Melting point | −60 °C (−76 °F; 213 K) |
| Boiling point | 102 to 103 °C (216 to 217 °F; 375 to 376 K) |
| Very slightly soluble | |
| Vapor pressure | 26 mmHg (20 °C)[3] |
| Hazards | |
| Flash point | 12 °C; 54 °F; 285 K[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[3] |
REL (Recommended)
|
TWA 50 ppm (175 mg/m3)[3] |
IDLH (Immediate danger)
|
N.D.[3] |
| Related compounds | |
Related aldehydes
|
Butyraldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
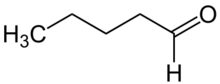
Pentanal (also called valeraldehyde) is the organic compound with molecular formula C
4H
9CHO. Classified as an alkyl aldehyde, it is a colorless volatile liquid. Its odor is described as fermented, bready, fruity, nutty, berry.[4]
Production
Pentanal is obtained by hydroformylation of butene. Also C4 mixtures can be used as starting material like the so-called raffinate II, which is produced by steam cracking and contains (Z)- and (E)-2-butene, 1-butene, butane and isobutane. The conversion to the product is accomplished with synthesis gas in the presence of a catalyst consisting of a rhodium-bisphosphite complex and a sterically hindered secondary amine with a selectivity toward pentanal of at least 90%.[5]
Use
Pentanal undergoes the reactions characteristic of any alkyl aldehyde, i.e., oxidations, condensations, and reductions. 2-Octanone, produced for use in the fragrance industry, is obtained by the condensation of acetone and pentanal, followed by hydrogenation of the alkene.[6]
2-Propyl-2-heptenal is obtained from pentanal by aldol condensation, which is hydrogenated to the saturated branched 2-propylheptanol. This alcohol serves as a starting material for the PVC plasticizer di-2-propylheptyl phthalate (DPHP).
Pentanal (valeraldehyde) is oxidized to give valeric acid.[7]
References
- ↑ Merck Index, 11th Edition, 9813.
- ↑ n-Valeraldehyde at chemicalland21.com
- ↑ 3.0 3.1 3.2 3.3 3.4 NIOSH Pocket Guide to Chemical Hazards. "#0652". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0652.html.
- ↑ "Valeraldehyde, 110-62-3". http://www.thegoodscentscompany.com/data/rw1009331.html.
- ↑ Patent WO 2009/146985 der Evonik Oxeno GmbH.
- ↑ Siegel, Hardo; Eggersdorfer, Manfred (2000). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_077. ISBN 9783527306732.
- ↑ Riemenschneider, Wilhelm (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_235.
 |

