Chemistry:Diethylmercury
From HandWiki
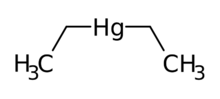
| |
| Names | |
|---|---|
| IUPAC name
diethylmercury
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | C007378 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H10Hg (C2H5)2Hg | |
| Molar mass | 258.71 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet |
| Density | 2.446 g/ml |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 156 to 157 °C (313 to 315 °F; 429 to 430 K) |
| Insoluble | |
| Solubility | Ethers, hydrocarbons, THF |
| Hazards | |
| Main hazards | Flammable, extremely toxic |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H225, H300+310+330Script error: No such module "Preview warning".Category:GHS errors, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P304+340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+233, P405, P501 | |
| Flash point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diethylmercury is a flammable, colorless liquid, and one of the strongest known neurotoxins. This organomercury compound is described as having a slightly sweet smell, though inhaling enough fumes to notice this would be hazardous.[1] This chemical can cross the blood–brain barrier, causing permanent brain damage. It is, however, considerably less toxic than dimethylmercury.
Synthesis
Diethylmercury can be obtained from the reaction between ethylmagnesium bromide and mercury(II) chloride.[2]
- 2 C2H5MgBr + HgCl2 → Hg(C2H5)2 + MgBr2 + MgCl2
Other methods are also known.[3]
See also
- Dimethylmercury, a related compound
- Ethylmercury
- Mercury poisoning
References
- ↑ "Diethyl Mercury | 627-44-1". http://www.chemicalbook.com/ChemicalProductProperty_EN.aspx?CBNumber=CB7165894.
- ↑ Brauer, Georg. Handbuch der präparativen anorganischen Chemie Bd. 2.. Baudler, Marianne (3rd ed.). Stuttgart. pp. 1063. ISBN 978-3-432-87813-3. OCLC 310719490. https://www.worldcat.org/oclc/310719490.
- ↑ Kolbe, Hermann (1860). Ausführliches Lehrbuch der organischen Chemie, Volume 2. pp. 964. https://books.google.com/books?id=-qU5AAAAcAAJ&pg=PA964.
 |

