Biology:Bacillus virus phi29
| Bacillus virus Φ29 | |
|---|---|
| |
| An illustration of Φ29's head based on electron microscopy data EMDB-2162 | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Duplodnaviria |
| Kingdom: | Heunggongvirae |
| Phylum: | Uroviricota |
| Class: | Caudoviricetes |
| Order: | Caudovirales |
| Family: | Salasmaviridae |
| Genus: | Salasvirus |
| Species: | Bacillus virus Φ29
|
File:Bacteriophage phi29.tif Bacillus virus Φ29 (bacteriophage Φ29) is a double-stranded DNA (dsDNA) bacteriophage with a prolate icosahedral head and a short tail that belongs to the genus Salasvirus, order Caudovirales, and family Salasmaviridae.[2][3] They are in the same order as phages PZA, Φ15, BS32, B103, M2Y (M2), Nf, and GA-1.[4][5] First discovered in 1965, the Φ29 phage is the smallest Bacillus phage isolated to date and is among the smallest known dsDNA phages.[2][3]
Φ29 has a unique DNA packaging motor structure that employs prohead packaging RNA (pRNA) to guide the translocation of the phage genome during replication. This novel structure system has inspired ongoing research in nanotechnology, drug delivery, and therapeutics.[6][7][8][9]
In nature, the Φ29 phage infects Bacillus subtilis, a species of gram-positive, endospore-forming bacteria that is found in soil, as well as the gastrointestinal tracts of various marine and terrestrial organisms, including human beings.[10]
History
In 1965, United States microbiologist Dr. Bernard Reilly discovered the Φ29 phage in Dr. John Spizizen’s lab at the University of Minnesota.[11][12] Due to its small size and complex morphology, it has become an ideal model for the study of many processes in molecular biology, such as morphogenesis, viral DNA packaging, viral replication, and transcription.[12][13]
Structure
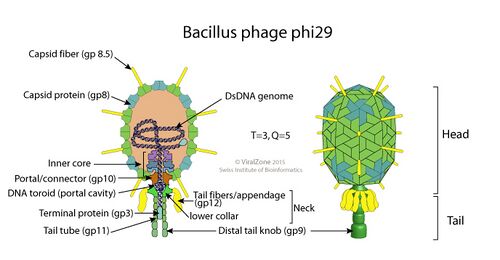
The structure of Φ29 is composed of seven main proteins: the terminal protein (p3), the head or capsid protein (p8), the head or capsid fiber protein (p8.5), the distal tail knob (p9), the portal or connector protein (p10), the tail tube or lower collar proteins (p11), and the tail fibers or appendage proteins (p12*).[6]
The main difference between Φ29's structure and that of other phages is its use of pRNA in its DNA packaging motor.[6]
DNA packaging motor
The Φ29 DNA packaging motor packages the phage genome into the procapsid during viral replication.[6] The Φ29 packaging motor is structurally composed of the procapsid and the connector proteins, which interact with the pRNA, the packaging enzyme (gp16), and the packaging substrate (genomic DNA-gp3).[6] Because the process of genome packaging is energy-intensive, it must be facilitated by an ATP-powered motor that converts chemical energy to mechanical energy through ATP hydrolysis.[6][14] The Φ29 packaging motor is able to generate approximately 57 piconewtons (pN) of force, making it one of the most powerful biomotors studied to date.[6]
pRNA
The Φ29 pRNA is a highly versatile molecule that can polymerize into dimers, trimers, tetramers, pentamers, and hexamers.[15] Early studies such as Anderson (1990) and Trottier (1998) hypothesized that pRNA formed intermolecular hexamers, but these studies had a solely genetic basis rather than a microscopy based approach.[16][17][18] In the year 2000, a study by Simpson et al. employed cryo-electron microscopy to determine that, in vivo, only a pentamer or smaller polymer could spatially fit in the virus.[18] Ultimately, single isomorphous replacement with anomalous scattering (SIRAS) crystallography was used to determine that the in vivo structure is a tetramer ring.[19] This discovery aligned with what was known about the structural geometry and necessary flexibility of the packaging motor’s three-way junction.[19] When pRNA is in this tetramer ring form, it works as a part of the DNA packaging motor to transport DNA molecules to their destination location within the prohead capsule.[20] Specifically, the functional domains of pRNA bind to the gp16 packaging enzyme and the structural connector molecule to aid in the translocation of DNA through the prohead channel.[6] After DNA packaging is complete, the pRNA dissociates and is degraded.[21]
Genome and replication
The Φ29 phage has a linear dsDNA genome consisting of 19,285 bases.[2] Both 5’ ends of the genome are capped with a covalently bonded terminal protein (p3) that complexes with DNA polymerase during replication.[2][22]
Φ29 is one of many phages with a DNA polymerase that has a different structure and function compared to standard DNA polymerases in other organisms.[22] Φ29 forms a replication complex involving the p3 terminal protein, the dAMP nucleotide, and its own DNA polymerase to synthesize DNA in a 5’ to 3’ direction. This replication process also employs a sliding-back mechanism towards the 3’ end of the genome that uses a repeating TTT motif to move the replication complex backward without altering the template sequence.[22][23] This allows the initiation of DNA replication to be more accurate by having the polymerase complex check a specific sequence before beginning the elongation process.[23][24]
Applications
Nanoparticle assembly
Versatility in RNA structure and function provides the ability to assemble nanoparticles for nanomedicinal therapeutics.[7] The pRNA in bacteriophage Φ29 can use its three-way junction in order to self-assemble into nanoparticles.[7]
One major challenge of using pRNA-derived nanoparticles is large-scale production, as most industries are currently unequipped to handle industrial pRNA synthesis.[8] This is primarily because RNA nanotechnology is still an emerging field that lacks industrial application and manufacturing optimization of small RNAs.[25]
Drug delivery
Φ29’s DNA packaging system, using pRNA, incorporates a motor for the delivery of therapeutic molecules like ribozymes and aptamers.[8] The small size of pRNA-derived nanoparticles also helps to deliver drugs in tight spaces like blood vessels.[8]
The main difficulty in using aptamer-based drug delivery is sourcing unique aptamers and other multimers for specific treatments for diseases that potentially degrade therapeutic multimers and nanoparticles in vivo.[8] Nanoparticles need to be stabilized as delivery mechanisms in order to adapt to microenvironments that may result in loss of therapeutic cargo.[26]
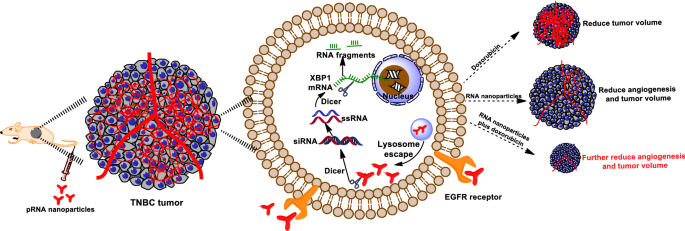
Triple-negative breast cancer treatment
Triple-negative breast cancer (TNBC) is an aggressive form of breast cancer that accounts for ten to fifteen percent of all breast cancer cases.[27] Chemotherapy is the only viable current treatment for TNBC because the loss of target receptors inherent to the disease causes cancer cells to resist therapeutic pharmaceuticals.[9]
The three-way junction in the Φ29 DNA packaging motor can help sensitize TNBC cells to chemotherapy using a siRNA drug delivery mechanism to inhibit TNBC growth and volume.[9] This treatment can also be combined with anti-cancer drugs like Doxorubicin to enhance therapeutic effects.[9]
See also
References
- ↑ Padilla-Sanchez, Victor (2021-07-17), Bacteriophage Φ29 structural model at atomic resolution, doi:10.5281/zenodo.5111609, https://zenodo.org/record/5111609, retrieved 2021-07-17
- ↑ 2.0 2.1 2.2 2.3 Meijer, Wilfried J. J.; Horcajadas, José A.; Salas, Margarita (2001). "φ29 Family of Phages". Microbiology and Molecular Biology Reviews 65 (2): 261–287. doi:10.1128/MMBR.65.2.261-287.2001. ISSN 1092-2172. PMID 11381102.
- ↑ 3.0 3.1 Ackermann, Hans-W. (1998). "Tailed Bacteriophages: The Order Caudovirales". Advances in Virus Research 51: 135–201. doi:10.1016/S0065-3527(08)60785-X. ISBN 9780120398515. ISSN 0065-3527. PMID 9891587.
- ↑ Bacteriophage : genetics and molecular biology. Stephen Mc Grath, Douwe van Sinderen. Norfolk, UK: Caister Academic Press. 2007. ISBN 978-1-904455-14-1. OCLC 86168751. https://www.worldcat.org/oclc/86168751.
- ↑ Camacho, Ana; Jimenez, Fernando; Torre, Javier; Carrascosa, Jose L.; Mellado, Rafael P.; Vinuela, Eladio; Salas, Margarita; Vasquez, Cesar (February 1977). "Assembly of Bacillus subtilis Phage Phi29. 1. Mutants in the Cistrons Coding for the Structural Proteins" (in en). European Journal of Biochemistry 73 (1): 39–55. doi:10.1111/j.1432-1033.1977.tb11290.x. ISSN 0014-2956. PMID 402269.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Lee, Tae Jin; Schwartz, Chad; Guo, Peixuan (2009-10-01). "Construction of Bacteriophage Phi29 DNA Packaging Motor and its Applications in Nanotechnology and Therapy" (in en). Annals of Biomedical Engineering 37 (10): 2064–2081. doi:10.1007/s10439-009-9723-0. ISSN 1573-9686. PMID 19495981. PMC 2855900. https://doi.org/10.1007/s10439-009-9723-0.
- ↑ 7.0 7.1 7.2 Shu, Yi; Wang, Hongzhi; Seremi, Bahar; Guo, Peixuan (2022), "Fabrication Methods for RNA Nanoparticle Assembly Based on Bacteriophage Phi29 pRNA Structural Features", RNA Nanotechnology and Therapeutics: pp. 141–157, doi:10.1201/9781003001560-21, ISBN 9781003001560, https://www.taylorfrancis.com/chapters/edit/10.1201/9781003001560-21/fabrication-methods-rna-nanoparticle-assembly-based-bacteriophage-phi29-prna-structural-features-yi-shu-hongzhi-wang-bahar-seremi-peixuan-guo, retrieved 2022-11-01
- ↑ 8.0 8.1 8.2 8.3 8.4 Ye, Xin; Hemida, Maged; Zhang, Huifang M.; Hanson, Paul; Ye, Qiu; Yang, Decheng (2012). "Current advances in Phi29 pRNA biology and its application in drug delivery: Current advances in Phi29 pRNA biology and its application" (in en). Wiley Interdisciplinary Reviews: RNA 3 (4): 469–481. doi:10.1002/wrna.1111. PMID 22362726.
- ↑ 9.0 9.1 9.2 9.3 Zhang, Long; Mu, Chaofeng; Zhang, Tinghong; Yang, Dejun; Wang, Chenou; Chen, Qiong; Tang, Lin; Fan, Luhui et al. (2021-01-07). "Development of targeted therapy therapeutics to sensitize triple-negative breast cancer chemosensitivity utilizing bacteriophage phi29 derived packaging RNA". Journal of Nanobiotechnology 19 (1): 13. doi:10.1186/s12951-020-00758-4. ISSN 1477-3155. PMID 33413427.
- ↑ Errington, Jeffery; van der Aart, Lizah T (2020-05-11). "Microbe Profile: Bacillus subtilis: model organism for cellular development, and industrial workhorse". Microbiology 166 (5): 425–427. doi:10.1099/mic.0.000922. ISSN 1350-0872. PMID 32391747.
- ↑ Reilly, Bernard E.; Spizizen, John (1965). "Bacteriophage Deoxyribonucleate Infection of Competent Bacillus subtilis1". Journal of Bacteriology 89 (3): 782–790. doi:10.1128/jb.89.3.782-790.1965. ISSN 0021-9193. PMID 14273661.
- ↑ 12.0 12.1 Salas, Margarita (2007-10-01). "40 Years with Bacteriophage ø29" (in en). Annual Review of Microbiology 61 (1): 1–22. doi:10.1146/annurev.micro.61.080706.093415. ISSN 0066-4227. PMID 17441785.
- ↑ "About | Virology". https://virology.umn.edu/about.
- ↑ Rao, Venigalla B.; Feiss, Michael (2008). "The bacteriophage DNA packaging motor". Annual Review of Genetics 42: 647–681. doi:10.1146/annurev.genet.42.110807.091545. ISSN 0066-4197. PMID 18687036. https://pubmed.ncbi.nlm.nih.gov/18687036/.
- ↑ Hoeprich, Stephen; Guo, Peixuan (2002-06-07). "Computer Modeling of Three-dimensional Structure of DNA-packaging RNA (pRNA) Monomer, Dimer, and Hexamer of Phi29 DNA Packaging Motor*" (in en). Journal of Biological Chemistry 277 (23): 20794–20803. doi:10.1074/jbc.M112061200. ISSN 0021-9258. PMID 11886855.
- ↑ Grimes, Shelley; Anderson, Dwight (1990-10-20). "RNA dependence of the bacteriophage φ29 DNA packaging ATPase" (in en). Journal of Molecular Biology 215 (4): 559–566. doi:10.1016/S0022-2836(05)80168-8. ISSN 0022-2836. PMID 1700132. https://www.sciencedirect.com/science/article/pii/S0022283605801688.
- ↑ Guo, Peixuan; Zhang, Chunlin; Chen, Chaoping; Garver, Kyle; Trottier, Mark (1998-07-01). "Inter-RNA Interaction of Phage φ29 pRNA to Form a Hexameric Complex for Viral DNA Transportation" (in English). Molecular Cell 2 (1): 149–155. doi:10.1016/S1097-2765(00)80124-0. ISSN 1097-2765. PMID 9702202.
- ↑ 18.0 18.1 Simpson, Alan A.; Tao, Yizhi; Leiman, Petr G.; Badasso, Mohammed O.; He, Yongning; Jardine, Paul J.; Olson, Norman H.; Morais, Marc C. et al. (2000). "Structure of the bacteriophage φ29 DNA packaging motor" (in en). Nature 408 (6813): 745–750. doi:10.1038/35047129. ISSN 1476-4687. PMID 11130079. Bibcode: 2000Natur.408..745S.
- ↑ 19.0 19.1 Ding, Fang; Lu, Changrui; Zhao, Wei; Rajashankar, Kanagalaghatta R.; Anderson, Dwight L.; Jardine, Paul J.; Grimes, Shelley; Ke, Ailong (2011-05-03). "Structure and assembly of the essential RNA ring component of a viral DNA packaging motor" (in en). Proceedings of the National Academy of Sciences 108 (18): 7357–7362. doi:10.1073/pnas.1016690108. ISSN 0027-8424. PMID 21471452. Bibcode: 2011PNAS..108.7357D.
- ↑ Guo, Peixuan; Zhang, Chunlin; Chen, Chaoping; Garver, Kyle; Trottier, Mark (1998-07-01). "Inter-RNA Interaction of Phage φ29 pRNA to Form a Hexameric Complex for Viral DNA Transportation" (in en). Molecular Cell 2 (1): 149–155. doi:10.1016/S1097-2765(00)80124-0. ISSN 1097-2765. PMID 9702202.
- ↑ Rao, Venigalla B.; Feiss, Michael (2015-11-09). "Mechanisms of DNA Packaging by Large Double-Stranded DNA Viruses" (in en). Annual Review of Virology 2 (1): 351–378. doi:10.1146/annurev-virology-100114-055212. ISSN 2327-056X. PMID 26958920.
- ↑ 22.0 22.1 22.2 Morcinek-Orłowska, Joanna; Zdrojewska, Karolina; Węgrzyn, Alicja (2022). "Bacteriophage-Encoded DNA Polymerases—Beyond the Traditional View of Polymerase Activities" (in en). International Journal of Molecular Sciences 23 (2): 635. doi:10.3390/ijms23020635. ISSN 1422-0067. PMID 35054821.
- ↑ 23.0 23.1 De Vega, Miguel; Salas, Margarita (2011-09-26). "Chapter 9: Protein-Primed Replication of Bacteriophage Φ29 DNA". in Kusic-Tisma, Jelena (in en). DNA Replication and Related Cellular Processes. IntechOpen. pp. 179–206. ISBN 978-9533077758. https://www.researchgate.net/publication/221917303.
- ↑ Grimes, Shelley; Jardine, Paul J.; Anderson, Dwight (2002-01-01) (in en), Bacteriophage φ29 DNA packaging, Advances in Virus Research, 58, Academic Press, pp. 255–294, doi:10.1016/s0065-3527(02)58007-6, ISBN 9780120398584, PMID 12205781, https://www.sciencedirect.com/science/article/pii/S0065352702580076, retrieved 2022-10-24
- ↑ Jasinski, Daniel; Haque, Farzin; Binzel, Daniel W; Guo, Peixuan (2017-02-07). "Advancement of the Emerging Field of RNA Nanotechnology" (in en). ACS Nano 11 (2): 1142–1164. doi:10.1021/acsnano.6b05737. ISSN 1936-0851. PMID 28045501.
- ↑ Shu, Yi; Pi, Fengmei; Sharma, Ashwani; Rajabi, Mehdi; Haque, Farzin; Shu, Dan; Leggas, Markos; Evers, B. Mark et al. (2014). "Stable RNA nanoparticles as potential new generation drugs for cancer therapy". Advanced Drug Delivery Reviews 66: 74–89. doi:10.1016/j.addr.2013.11.006. ISSN 0169-409X. PMID 24270010.
- ↑ "Triple-negative Breast Cancer | Details, Diagnosis, and Signs" (in en). https://www.cancer.org/cancer/breast-cancer/about/types-of-breast-cancer/triple-negative.html.
Wikidata ☰ {{{from}}} entry
 |