Biology:Kringle domain
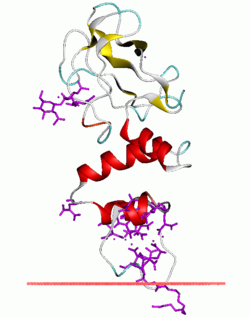 Fragment of bovine prothrombin in complex with calcium and lysophosphatidylserine. The protein associate with membrane through its alpha-helical GLA domain. The adjacent kringle domain is beta-structural (yellow). | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Kringle | ||||||||
| Pfam | PF00051 | ||||||||
| InterPro | IPR000001 | ||||||||
| SMART | KR | ||||||||
| PROSITE | PDOC00020 | ||||||||
| SCOP2 | 1pk4 / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 115 | ||||||||
| OPM protein | 1h8p | ||||||||
| CDD | cd00108 | ||||||||
| |||||||||
Kringle domains are autonomous protein domains that fold into large loops stabilized by 3 disulfide linkages. These are important in protein–protein interactions with blood coagulation factors. Their name refers to the Kringle, a Scandinavian pastry which they somewhat resemble.
Kringle domains have been found in plasminogen, hepatocyte growth factors, prothrombin, and apolipoprotein(a).
Kringles are found throughout the blood clotting and fibrinolytic proteins. Kringle domains are believed to play a role in binding mediators (e.g., membranes, other proteins or phospholipids), and in the regulation of proteolytic activity.[1][2][3] Kringle domains[4][5][6] are characterised by a triple loop, 3-disulfide bridge structure, whose conformation is defined by a number of hydrogen bonds and small pieces of anti-parallel beta-sheet. They are found in a varying number of copies in some plasma proteins including prothrombin and urokinase-type plasminogen activator, which are serine proteases belonging to MEROPS peptidase family S1A.
Human proteins containing this domain
ATF; F12; F2; HABP2; HGF; HGFAC; KREMEN1; KREMEN2; LPA; LPAL2; MST1; PIK3IP1; PLAT; PLAU; PLG; PRSS12; ROR1; ROR2;
References
- ↑ "Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor)". J. Biol. Chem. 260 (9): 5328–5341. 1985. doi:10.1016/S0021-9258(18)89026-3. PMID 3886654.
- ↑ "Kringles: modules specialized for protein binding. Homology of the gelatin-binding region of fibronectin with the kringle structures of proteases". FEBS Lett. 171 (1): 131–136. 1984. doi:10.1016/0014-5793(84)80473-1. PMID 6373375.
- ↑ "Solution structure of the kringle 4 domain from human plasminogen by 1H nuclear magnetic resonance spectroscopy and distance geometry". J. Mol. Biol. 212 (3): 541–552. 1990. doi:10.1016/0022-2836(90)90330-O. PMID 2157850.
- ↑ "The genetic relationships between the kringle domains of human plasminogen, prothrombin, tissue plasminogen activator, urokinase, and coagulation factor XII". J. Mol. Evol. 26 (4): 358–369. 1987. doi:10.1007/BF02101155. PMID 3131537. Bibcode: 1987JMolE..26..358C.
- ↑ Patthy L (1985). "Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules". Cell 41 (3): 657–663. doi:10.1016/S0092-8674(85)80046-5. PMID 3891096.
- ↑ "Evolutionary origin of numerous kringles in human and simian apolipoprotein(a)". FEBS Lett. 287 (1): 146–148. 1991. doi:10.1016/0014-5793(91)80036-3. PMID 1879523.
External links
- Kringle domain in PROSITE
- KR domain entry in the SMART database
- Kringle domain cartoon, under Prothrombin Structure
 |
