Biology:Factor XII
 Generic protein structure example |
Coagulation factor XII, also known as Hageman factor, is a plasma protein. It is the zymogen form of factor XIIa, an enzyme (EC 3.4.21.38) of the serine protease (or serine endopeptidase) class. In humans, factor XII is encoded by the F12 gene.[1]
Structure
Human Factor XII is 596 amino acids long and consists of two chains, the heavy chain (353 residues) and light chain (243 residues) held together by a disulfide bond. It is 80,000 daltons. Its heavy chain contains two fibronectin-type domains (type I and II), two epidermal growth factor-like domains, a kringle domain, and a proline-rich region, and its light chain contains the protease domain. The structure of the FnI-EGF-like tandem domain of coagulation factor XII has been solved by X-ray crystallography.[2][3] Crystal structures of the FXII light chain has also been determined unbound (β-FXII) and bound (β-FXIIa) to inhibitors.[4][5][6]
Factor XII (FXII, Hageman factor) is a plasma glycoprotein of approximately 90 kDa molecular weight is part of the coagulation cascade and activates factor XI and prekallikrein in vitro. Factor XII itself is activated to factor XIIa by negatively charged surfaces, such as glass. This is the starting point of the intrinsic pathway.[7] Factor XII can also be used to start coagulation cascades in laboratory diagnostic coagulation assays called activated partial thromboplastin times (aPTT).[8]
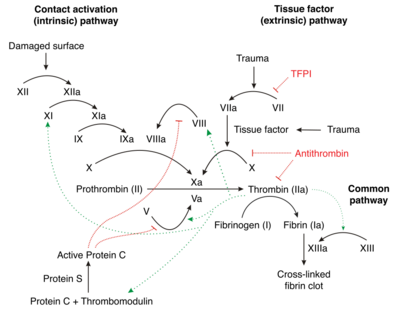
In vivo, factor XII is activated by binding (contact) to polyanions termed contact-activation. Multiple polymers, the white clay material kaolin and glass are non-physiological factor XII contact activators. Activated platelets release inorganic polymers, polyphosphates. Contact to polyphosphates activates factor XII and initiates fibrin formation by the intrinsic pathway of coagulation with critical importance for thrombus formation and the factor XII-activated pro inflammatory kallikrein kinin-system. Targeting polyphosphates with phosphatases interfered with procoagulant activity of activated platelets and blocked platelet-induced thrombosis in mice. Addition of polyphosphates restored defective plasma clotting of Hermansky–Pudlak syndrome patients, indicating that the inorganic polymer is the endogenous factor XII activator in vivo. Platelet polyphosphate-driven factor XII activation provides the link from primary hemostasis (formation of a platelet plug) to secondary hemostasis (fibrin meshwork formation).[9] Polyphosphate exerts differential effects on plasma clotting in test tubes ex vivo, depending on polymer size and it was shown in vitro that platelet-size soluble polyphosphates induce little activaton of factor XII in solution but that they are accelerators of thrombin-induced activation of factor XI.[10] The mystery was solved upon the discovery that short chain polyphosphate forms insoluble calcium-rich nanoparticles in vivo. These aggregates accumulate on the platelet surface and activate factor XII independently of the chain length of the individual polymer.[11] Regulation of polyphosphates in platelets has remained poorly understood. Combinations of systems biology, genetics and functional analyses has identified the phosphate-exporter XPR1 as important regulator of polyphosphates in platelets. Targeting XPR1 increases polyphosphate content and leads to accelerated arterial and venous thrombosis in mouse models.[12]
Based on the seminar role of factor XII in thrombosis while sparing haemostats, targeting the protease has emerged as a promising drug target for safe anticoagulant drugs that in contrast to currently used anticoagulants, do not increase bleeding. Multiple factor XII inhibitors have been developed and some of them are in clinical trials [13]
Genetics
The gene for factor XII is located on the tip of the long arm of the fifth chromosome (5q33-qter).[1]
Role in disease
Factor XII deficiency is a rare disorder that is inherited in an autosomal recessive manner.[14] Unlike other clotting factor deficiencies, factor XII deficiency is totally asymptomatic and does not cause excess bleeding.[14] Mice lacking the gene for factor XII, however, are less susceptible to thrombosis. The protein seems to be involved in the later stages of clot formation rather than the first occlusion of damages in the blood vessel wall.[15]
Factor XII does play an important role in clot formation during in vitro measurements of the partial thromboplastin time, which causes these measurements to be markedly prolonged in patients with factor XII deficiency, usually well beyond even what is seen in hemophilia A, hemophilia B, or factor XI deficiency.[14] As a result, the main concern related to factor XII deficiency is the unnecessary testing, delay in care, worry, etc. that may be prompted by the abnormal lab result.[14] All of this, including the mechanism of inheritance, also holds true for the other contact factors, prekallikrein (Fletcher factor) and high molecular weight kininogen.[14]
Excess levels of factor XII can predispose individuals towards greater risk of venous thrombosis due to factor XII's role as one of the catalysts for conversion of plasminogen to its active fibrinolytic form of plasmin.[16]
Factor XII is also activated by endotoxins, especially lipid A in vitro.
Experimental mouse models have suggested a role of FXII in multiple sclerosis.[17]
History
Hageman factor was first discovered in 1955 when a routine preoperative blood sample of the 37-year-old railroad brakeman John Hageman (1918) was found to have prolonged clotting time in test tubes, even though he had no hemorrhagic symptoms. Hageman was then examined by hematologist Oscar Ratnoff, who found that Hageman lacked a previously unidentified clotting factor.[18] Ratnoff later found that the Hageman factor deficiency is an autosomal recessive disorder, after examining several related people who had the deficiency. Paradoxically, pulmonary embolism contributed to Hageman's death after an occupational accident in 1968. Since then, case studies and clinical studies identified an association between thrombosis and Factor XII deficiency. Hepatocytes express blood coagulation factor XII.[19]
Currently produced QuikClot products, produced and marketed primarily for use in battlefield medicine to treat penetrating trauma (such as gunshot wounds and stab wounds), and other injuries that are known to commonly cause exsanguination (such as blast injury), are used with the overarching goal of increasing the time between the blood loss occurring, and the patient succumbing to the blood loss. The purpose of increasing this time is so that the patient may reaching a higher level of medical care before succumbing from their injuries. These products use a Kaolinite-based coating, applied to the bandages by the manufacturer before packaging and sale. This coating, when applied to an open wound via the application of the bandages, directly promotes blood clotting by activating Factor XII in the coagulation cascade.[20] Also, due to the active ingredient nature of Kaolinite, the activation of the Factor XII occurs in both an earlier amount of time than it otherwise would, and at an increased, more rapid rate than it otherwise would.[21][22] This coating is widely considered amongst combat medics to be vastly superior to the older QuikClot powder formulation, which was poured into wounds, due to the fact that the older formulation used bead-form Zeolite, a mineral which promotes the coagulation cascade, due to the fact that the reaction between the Zeolite powder and the blood inside the wound site was an Exothermic one, sometimes so intensely that it caused cases of second degree burns on the inside surface of the wound. This, obviously, caused extreme pain to the patient, often more-so than the initial injury was causing them at the time (assuming the patient was still conscious at the time of the application of the powder).[23] This effect is often seen in movies and TV programs, with the QuikClot powder being poured into wounds, and the patient screaming out in pain as their wounds were violently burned on the inside surface of the wounds. This created a common misconception, which persists to this day, that commonly used QuikClot products still use this method of clot promotion (Zeolite powder) to this day. However, Zeolite-based clotting products are no longer widely used by militaries and police departments throughout the western world, as they have been widely supplanted by the Kaolinite-based bandage products, which do not cause any exothermic reaction whatsoever, nor do they have the absolute-requirement of the application of the product exclusively to the inside-surface of the wound.
References
- ↑ 1.0 1.1 "Characterization of the human blood coagulation factor XII gene. Intron/exon gene organization and analysis of the 5'-flanking region". The Journal of Biological Chemistry 262 (28): 13662–13673. October 1987. doi:10.1016/S0021-9258(19)76478-3. PMID 2888762.
- ↑ "Factor XII: what does it contribute to our understanding of the physiology and pathophysiology of hemostasis & thrombosis". Thrombosis Research 125 (3): 210–215. March 2010. doi:10.1016/j.thromres.2009.11.028. PMID 20022081.
- ↑ "The structure of the FnI-EGF-like tandem domain of coagulation factor XII solved using SIRAS". Acta Crystallographica Section F 69 (Pt 2): 94–102. February 2013. doi:10.1107/S1744309113000286. PMID 23385745.
- ↑ "Structures of human plasma β-factor XIIa cocrystallized with potent inhibitors". Blood Advances 2 (5): 549–558. March 2018. doi:10.1182/bloodadvances.2018016337. PMID 29519898.
- ↑ "Coagulation factor XII protease domain crystal structure". Journal of Thrombosis and Haemostasis 13 (4): 580–591. April 2015. doi:10.1111/jth.12849. PMID 25604127.
- ↑ "Crystal structures of the recombinant β-factor XIIa protease with bound Thr-Arg and Pro-Arg substrate mimetics". Acta Crystallographica Section D 75 (Pt 6): 578–591. June 2019. doi:10.1107/S2059798319006910. PMID 31205020. https://nottingham-repository.worktribe.com/output/2390798.
- ↑ Textbook of Medical Physiology (11th ed.). pp. 462–463. ISBN 0-7216-0240-1. https://www.moscmm.org/pdf/Guyton%20physiology.pdf. Retrieved 2021-01-25.
- ↑ "In vivo roles of factor XII". Blood 120 (22): 4296–4303. November 2012. doi:10.1182/blood-2012-07-292094. PMID 22993391.
- ↑ "Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo". Cell 139 (6): 1143–1156. December 2009. doi:10.1016/j.cell.2009.11.001. PMID 20005807.
- ↑ "Polyphosphate exerts differential effects on blood clotting, depending on polymer size". Blood 116 (20): 4353–4359. November 2010. doi:10.1182/blood-2010-01-266791. PMID 20709905.
- ↑ "Polyphosphate nanoparticles on the platelet surface trigger contact system activation". Blood 129 (23): 1707–1717. January 2017. doi:10.1182/blood-2016-08-734988. PMID 28049643.
- ↑ Mailer, R. K.; Allende, M.; Heestermans, M.; Schweizer, M.; Deppermann, C.; Frye, M.; Pula, G.; Odeberg, J. et al. (2021). "Xenotropic and polytropic retrovirus receptor 1 regulates procoagulant platelet polyphosphate". Blood 137 (10): 1392–1405. doi:10.1182/blood.2019004617. PMID 32932519.
- ↑ Davoine, C.; Bouckaert, C.; Fillet, M.; Pochet, L. (2020). "Factor XII/XIIa inhibitors: Their discovery, development, and potential indications". European Journal of Medicinal Chemistry 208: 112753. doi:10.1016/j.ejmech.2020.112753. PMID 32883641. https://pubmed.ncbi.nlm.nih.gov/32883641/.
- ↑ 14.0 14.1 14.2 14.3 14.4 "The laboratory approach to inherited and acquired coagulation factor deficiencies". Clinics in Laboratory Medicine 29 (2): 229–252. June 2009. doi:10.1016/j.cll.2009.04.002. PMID 19665676. https://zenodo.org/record/894920.
- ↑ "Defective thrombus formation in mice lacking coagulation factor XII". The Journal of Experimental Medicine 202 (2): 271–281. July 2005. doi:10.1084/jem.20050664. PMID 16009717.
- ↑ Manual of Coagulation Disorders. Blackwell Science. 2001. pp. 3–4, 206–207. ISBN 0-86542-446-2.
- ↑ "Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells". Nature Communications 7: 11626. May 2016. doi:10.1038/ncomms11626. PMID 27188843. Bibcode: 2016NatCo...711626G.
- ↑ "Hageman trait: an asymptomatic disorder of blood coagulation". Transactions of the Association of American Physicians 68: 149–154. 1955. PMID 13299324.
- ↑ "Hepatocytes express blood coagulation factor XII (Hageman factor)". The Journal of Laboratory and Clinical Medicine 115 (4): 463–469. April 1990. PMID 2324612.
- ↑ Tissue-biomaterial interactions.. Hoboken: Wiley & Sons. 2002. ISBN 978-0-471-46112-8.
- ↑ "A new kaolin-based haemostatic bandage compared with manual compression for bleeding control after percutaneous coronary procedures". European Radiology 21 (8): 1687–1691. August 2011. doi:10.1007/s00330-011-2117-3. PMID 21476127.
- ↑ "Randomized clinical trial on short-time compression with Kaolin-filled pad: a new strategy to avoid early bleeding and subacute radial artery occlusion after percutaneous coronary intervention". Journal of Interventional Cardiology 24 (1): 65–72. February 2011. doi:10.1111/j.1540-8183.2010.00584.x. PMID 20807305.
- ↑ "Thermal injury resulting from application of a granular mineral hemostatic agent". The Journal of Trauma 57 (2): 224–230. August 2004. doi:10.1097/01.ta.0000105916.30158.06. PMID 15345965.
Further reading
- "The occasional venous thromboses seen in patients with severe (homozygous) FXII deficiency are probably due to associated risk factors: a study of prevalence in 21 patients and review of the literature". Journal of Thrombosis and Thrombolysis 17 (2): 139–143. April 2004. doi:10.1023/B:THRO.0000037670.42776.cd. PMID 15306750.
- "Role of Factor XII in hemostasis and thrombosis: clinical implications". Expert Review of Cardiovascular Therapy 5 (4): 733–741. July 2007. doi:10.1586/14779072.5.4.733. PMID 17605651.
- "O-linked fucose is present in the first epidermal growth factor domain of factor XII but not protein C". The Journal of Biological Chemistry 267 (8): 5102–5107. March 1992. doi:10.1016/S0021-9258(18)42736-6. PMID 1544894.
- "Location of the disulfide bonds in human plasma prekallikrein: the presence of four novel apple domains in the amino-terminal portion of the molecule". Biochemistry 30 (8): 2050–2056. February 1991. doi:10.1021/bi00222a007. PMID 1998666.
- "Coagulation factor XII (Hageman factor) Washington D.C.: inactive factor XIIa results from Cys-571----Ser substitution". Proceedings of the National Academy of Sciences of the United States of America 86 (21): 8319–8322. November 1989. doi:10.1073/pnas.86.21.8319. PMID 2510163. Bibcode: 1989PNAS...86.8319M.
- "Factor XII gene alteration in Hageman trait detected by TaqI restriction enzyme". Blood 69 (5): 1421–1424. May 1987. doi:10.1182/blood.V69.5.1421.1421. PMID 2882793.
- "Characterization of the human blood coagulation factor XII gene. Intron/exon gene organization and analysis of the 5'-flanking region". The Journal of Biological Chemistry 262 (28): 13662–13673. October 1987. doi:10.1016/S0021-9258(19)76478-3. PMID 2888762.
- "Characterization of a cDNA coding for human factor XII (Hageman factor)". Biochemistry 25 (7): 1525–1528. April 1986. doi:10.1021/bi00355a009. PMID 3011063.
- "Structural gene encoding human factor XII is located at 5q33-qter". Somatic Cell and Molecular Genetics 14 (2): 217–221. March 1988. doi:10.1007/BF01534407. PMID 3162339.
- "Assignment of human coagulation factor XII (fXII) to chromosome 5 by cDNA hybridization to DNA from somatic cell hybrids". Human Genetics 80 (4): 397–398. December 1988. doi:10.1007/BF00273661. PMID 3198120.
- "Inhibition of the activation of Hageman factor (factor XII) by beta 2-glycoprotein I". The Journal of Laboratory and Clinical Medicine 111 (5): 519–523. May 1988. PMID 3361230.
- "Human plasma prekallikrein, a zymogen to a serine protease that contains four tandem repeats". Biochemistry 25 (9): 2410–2417. May 1986. doi:10.1021/bi00357a017. PMID 3521732.
- "cDNA sequence coding for human coagulation factor XII (Hageman)". Nucleic Acids Research 14 (7): 3146. April 1986. doi:10.1093/nar/14.7.3146. PMID 3754331.
- "Characterization of human blood coagulation factor XII cDNA. Prediction of the primary structure of factor XII and the tertiary structure of beta-factor XIIa". The Journal of Biological Chemistry 260 (25): 13666–13676. November 1985. doi:10.1016/S0021-9258(17)38776-8. PMID 3877053.
- "Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor)". The Journal of Biological Chemistry 260 (9): 5328–5341. May 1985. doi:10.1016/S0021-9258(18)89026-3. PMID 3886654.
- "Tentative localization of a Hageman (Factor XII) locus on 7q, probably the 7q35 band". Humangenetik 24 (3): 197–200. 1975. doi:10.1007/bf00283584. PMID 4140832.
- "Amino acid sequence of human beta-factor XIIa". The Journal of Biological Chemistry 258 (18): 10924–10933. September 1983. doi:10.1016/S0021-9258(17)44364-X. PMID 6604055.
- "Coagulation factor XII Locarno: the functional defect is caused by the amino acid substitution Arg 353-->Pro leading to loss of a kallikrein cleavage site". Blood 84 (4): 1173–1181. August 1994. doi:10.1182/blood.V84.4.1173.1173. PMID 8049433.
- "The novel acceptor splice site mutation 11396(G-->A) in the factor XII gene causes a truncated transcript in cross-reacting material negative patients". Human Molecular Genetics 4 (7): 1235–1237. July 1995. doi:10.1093/hmg/4.7.1235. PMID 8528215.
- "A novel 5'-upstream mutation in the factor XII gene is associated with a TaqI restriction site in an Alu repeat in factor XII-deficient patients". Human Genetics 97 (6): 838–841. June 1996. doi:10.1007/BF02346200. PMID 8641707.
External links
- The MEROPS online database for peptidases and their inhibitors: S01.211
- Factor+XII at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P00748 (Coagulation factor XII) at the PDBe-KB.
 |
