Biology:Microsporidia
| Microsporidia | |
|---|---|
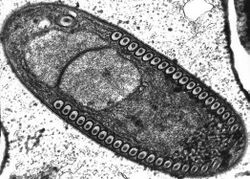
| |
| Sporoblast of Fibrillanosema crangonycis | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Obazoa |
| (unranked): | Opisthokonta |
| Clade: | Holomycota |
| Kingdom: | Fungi |
| Subkingdom: | Rozellomyceta |
| Phylum: | Rozellomycota |
| Class: | Microsporidea Corliss & Levine, 1963[1] |
| Orders[2] | |
| |
| Synonyms | |
Microsporidia are a group of spore-forming unicellular parasites. These spores contain an extrusion apparatus that has a coiled polar tube ending in an anchoring disc at the apical part of the spore.[7] They were once considered protozoans or protists, but are now known to be fungi,[8] or a sister group to fungi.[9] These fungal microbes are obligate eukaryotic parasites that use a unique mechanism to infect host cells.[7] They have recently been discovered in a 2017 Cornell study to infect Coleoptera on a large scale. So far, about 1500 of the probably more than one million[10] species are named. Microsporidia are restricted to animal hosts, and all major groups of animals host microsporidia. Most infect insects, but they are also responsible for common diseases of crustaceans and fish. The named species of microsporidia usually infect one host species or a group of closely related taxa. Approximately 10 percent of the species are parasites of vertebrates —several species, most of which are opportunistic, can infect humans, in whom they can cause microsporidiosis.
After infection they influence their hosts in various ways and all organs and tissues are invaded, though generally by different species of specialised microsporidia. Some species are lethal, and a few are used in biological control of insect pests. Parasitic castration, gigantism, or change of host sex are all potential effects of microsporidian parasitism (in insects). In the most advanced cases of parasitism the microsporidium rules the host cell completely and controls its metabolism and reproduction, forming a xenoma.[11]
Replication takes place within the host's cells, which are infected by means of unicellular spores. These vary from 1–40 μm, making them some of the smallest eukaryotes.[citation needed] Microsporidia that infect mammals are 1.0–4.0 μm.[12] They also have the smallest eukaryotic genomes.
The terms "microsporidium" (pl. "microsporidia") and "microsporidian" are used as vernacular names for members of the group. The name Microsporidium Balbiani, 1884[13] is also used as a catchall genus for incertae sedis members.[14]

Morphology
File:Parasite140027-fig5 Dictyocoela diporeiae Winters & Faisal, 2014 transmission electron micrographs.tif Microsporidia lack mitochondria, instead possessing mitosomes. They also lack motile structures, such as flagella.
Microsporidia produce highly resistant spores, capable of surviving outside their host for up to several years. Spore morphology is useful in distinguishing between different species. Spores of most species are oval or pyriform, but rod-shaped or spherical spores are not unusual. A few genera produce spores of unique shape for the genus.
The spore is protected by a wall, consisting of three layers:
- an outer electron-dense exospore
- a median, wide and seemingly structureless endospore, containing chitin
- a thin internal plasma membrane
In most cases there are two closely associated nuclei, forming a diplokaryon, but sometimes there is only one.
The anterior half of the spore contains a harpoon-like apparatus with a long, thread-like polar filament, which is coiled up in the posterior half of the spore. The anterior part of the polar filament is surrounded by a polaroplast, a lamella of membranes. Behind the polar filament, there is a posterior vacuole.[11]
Infection
In the gut of the host the spore germinates; it builds up osmotic pressure until its rigid wall ruptures at its thinnest point at the apex. The posterior vacuole swells, forcing the polar filament to rapidly eject the infectious content into the cytoplasm of the potential host. Simultaneously the material of the filament is rearranged to form a tube which functions as a hypodermic needle and penetrates the gut epithelium.
Once inside the host cell, a sporoplasm grows, dividing or forming a multinucleate plasmodium, before producing new spores. The life cycle varies considerably. Some have a simple asexual life cycle,[16] while others have a complex life cycle involving multiple hosts and both asexual and sexual reproduction. Different types of spores may be produced at different stages, probably with different functions including autoinfection (transmission within a single host).
Medical implications
In animals and humans, microsporidia often cause chronic, debilitating diseases rather than lethal infections. Effects on the host include reduced longevity, fertility, weight, and general vigor. Vertical transmission of microsporidia is frequently reported.
In the case of insect hosts, vertical transmission often occurs as transovarial transmission, where the microsporidian parasites pass from the ovaries of the female host into eggs and eventually multiply in the infected larvae. Amblyospora salinaria n. sp. which infects the mosquito Culex salinarius Coquillett, and Amblyospora californica which infects the mosquito Culex tarsalis Coquillett, provide typical examples of transovarial transmission of microsporidia.[17][18][19][20] Microsporidia, specifically the mosquito-infecting Vavraia culicis, are being explored as a possible 'evolution-proof' malaria-control method.[21] Microsporidian infection of Anopheles gambiae (the principal vector of Plasmodium falciparum malaria) reduces malarial infection within the mosquito, and shortens the mosquito lifespan.[22] As the majority of malaria-infected mosquitoes naturally die before the malaria parasite is mature enough to transmit, any increase in mosquito mortality through microsporidian-infection may reduce malaria transmission to humans. In May 2020, researchers reported that Microsporidia MB, a symbiont in the midgut and ovaries of An. arabiensis, significantly impaired transmission of P. falciparum, had "no overt effect" on the fitness of host mosquitoes, and was transmitted vertically (through inheritance).[23]
Clinical
Microsporidian infections of humans sometimes cause a disease called microsporidiosis. At least 14 microsporidian species, spread across eight genera, have been recognized as human pathogens. These include Trachipleistophora hominis.[24]
As hyperparasites
File:Parasite140019-fig4 Nosema podocotyloidis - Hyperparasitic Microsporidia.tif
Microsporidia can infect a variety of hosts, including hosts which are themselves parasites. In that case, the microsporidian species is a hyperparasite, i.e. a parasite of a parasite. As an example, more than eighteen species are known which parasitize digeneans (parasitic flatworms). These digeneans are themselves parasites in various vertebrates and molluscs. Eight of these species belong to the genus Nosema.[25] Similarly, the microsporidian species Toguebayea baccigeri is a parasite of a digenean, the faustulid Bacciger israelensis, itself an intestinal parasite of a marine fish, the bogue Boops boops (Teleostei, Sparidae).[26]
Genomes
Microsporidia have the smallest known (nuclear) eukaryotic genomes. The parasitic lifestyle of microsporidia has led to a loss of many mitochondrial and Golgi genes, and even their ribosomal RNAs are reduced in size compared with those of most eukaryotes. As a consequence, the genomes of microsporidia are much smaller than those of other eukaryotes. Currently known microsporidial genomes are 2.5 to 11.6 Mb in size, encoding from 1,848 to 3,266 proteins which is in the same range as many bacteria.[27]
Horizontal gene transfer (HGT) seems to have occurred many times in microsporidia. For instance, the genomes of Encephalitozoon romaleae and Trachipleistophora hominis contain genes that derive from animals and bacteria, and some even from fungi.[27]
DNA repair
The Rad9-Rad1-Hus1 protein complex (also known as the 9-1-1 complex) in eukaryotes is recruited to sites of DNA damage where it is considered to help trigger the checkpoint-signaling cascade. Genes that code for heterotrimeric 9-1-1 are present in microsporidia.[28] In addition to the 9-1-1 complex, other components of the DNA repair machinery are also present indicting that repair of DNA damage likely occurs in microsporidia.[28]
Classification
The first described microsporidian genus, Nosema, was initially put by Nägeli in the fungal group Schizomycetes together with some bacteria and yeasts.[29][30] For some time microsporidia were considered as very primitive eukaryotes, placed in the protozoan group Cnidospora.[1] Later, especially because of the lack of mitochondria, they were placed along with the other Protozoa such as diplomonads, parabasalids and archamoebae in the protozoan-group Archezoa.[31] More recent research has falsified this theory of early origin (for all of these). Instead, microsporidia are proposed to be highly developed and specialized organisms, which just dispensed functions that are needed no longer, because they are supplied by the host.[32] Furthermore, spore-forming organisms in general do have a complex system of reproduction, both sexual and asexual, which look far from primitive.
Since the mid-2000s microsporidia are placed within the Fungi or as a sister-group of the Fungi with a common ancestor.[33][34][35][36]
Work to identify clades is largely based on habitat and host. Three classes of Microsporidia are proposed by Vossbrinck and Debrunner-Vossbrinck, based on the habitat: Aquasporidia, Marinosporidia and Terresporidia.[37]
A second classification by Cavalier-Smith 1993:[38]
- Subphyla Rudimicrospora Cavalier-Smith 1993
- Class Minisporea Cavalier-Smith 1993
- Order Minisporida Sprague, 1972
- Class Metchnikovellea Weiser, 1977
- Order Metchnikovellida Vivier, 1975
- Class Minisporea Cavalier-Smith 1993
- Subphyla Polaroplasta Cavalier-Smith 1993
- Class Pleistophoridea Cavalier-Smith 1993
- Order Pleistophorida Stempell 1906
- Class Disporea Cavalier-Smith 1993
- Subclass Unikaryotia Cavalier-Smith 1993
- Subclass Diplokaryotia Cavalier-Smith 1993
- Class Pleistophoridea Cavalier-Smith 1993
|
See also
- List of Microsporidian genera
- Glugea, a genus of microsporidia
- Nosema apis, a microsporidian parasite of bees
References
- ↑ 1.0 1.1 "Establishment of the Microsporidea as a new class in the protozoan subphylum Cnidospora". The Journal of Protozoology 10 (Suppl): 26–27. 1963. doi:10.1111/jeu.1963.10.issue-s3.
- ↑ Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K. et al. (2022). "Outline of Fungi and fungus-like taxa – 2021". Mycosphere 13 (1): 53–453. doi:10.5943/mycosphere/13/1/2. https://www.researchgate.net/publication/358798332.
- ↑ Balbiani, G (1882). "Sur les microsporidies ou psorospermies des Articulés". C. R. Acad. Sci. 95: 1168–71. https://www.biodiversitylibrary.org/item/112005#page/1166/mode/1up.
- ↑ Delphy, J. 1936. Sous-règne des Protozoaires. In: Perrier, R. (ed.). La Faune de la France en tableaux synoptiques illustrés, vol 1A. Delagrave: Paris.
- ↑ Levine, N. D. (1980). "A Newly Revised Classification of the Protozoa". The Journal of Protozoology 27 (1): 37–58. doi:10.1111/j.1550-7408.1980.tb04228.x. PMID 6989987. https://www.researchgate.net/publication/16209026.
- ↑ Sprague, V. (1977). Classification and phylogeny of the Microsporidia. In: Comparative pathobiology. vol. 2, Systematics of the Microsporidia. Lee A. Bulla & Thomas C. Cheng (ed.). pp. 1–30. New York: Plenum Press, [1].
- ↑ 7.0 7.1 Franzen, C. (2005). How do Microsporidia invade cells?. Folia Parasitologica, 52(1–2), 36–40. doi.org/10.14411/fp.2005.005
- ↑ Hibbett, D.S. (2007). "A higher level phylogenetic classification of the Fungi". Mycological Research 111 (5): 509–47. doi:10.1016/j.mycres.2007.03.004. PMID 17572334. http://www.umich.edu/~mycology/resources/Publications/Hibbett-et-al.-2007.pdf.
- ↑ Silar, Philippe (2016). Protistes Eucaryotes : Origine, Evolution et Biologie des Microbes Eucaryotes. HAL. p. 462. ISBN 978-2-9555841-0-1. https://hal.archives-ouvertes.fr/hal-01263138/document.
- ↑ Hawksworth, David (2001). "The magnitude of fungal diversity: The 1.5 million spices estimate revisited". Mycological Research 105 (12): 1422. doi:10.1017/S0953756201004725.
- ↑ 11.0 11.1 Ronny Larsson, Lund University (Department of Cell and Organism Biology) Cytology and taxonomy of the microsporidia 2004.
- ↑ Didier, ES. (Apr 2005). "Microsporidiosis: an emerging and opportunistic infection in humans and animals". Acta Trop 94 (1): 61–76. doi:10.1016/j.actatropica.2005.01.010. PMID 15777637.
- ↑ Balbiani, G. 1884. Les Psorospermies des Articulés ou Microsporidies, pp. 150-168, 184. In: Leçons sur les sporozoaires. Paris: Doin, [2].
- ↑ Hoffman, G. (1999). Parasites of North American Freshwater Fishes, 2nd edn, University of California Press, Berkeley, California, USA, p. 89, [3].
- ↑ Winters, A. D.; Faisal, M. (2014). "Molecular and ultrastructural characterization of Dictyocoela diporeiae n. sp. (Microsporidia), a parasite of Diporeia spp. (Amphipoda, Gammaridea)". Parasite 21: 26. doi:10.1051/parasite/2014028. PMID 24934702.
- ↑ Ironside JE (2007). "Multiple losses of sex within a single genus of Microsporidia". BMC Evolutionary Biology 7: 48. doi:10.1186/1471-2148-7-48. PMID 17394631.
- ↑ "Development, ultrastructure, and mode of transmission of Amblyospora sp. (Microspora) in the mosquito". The Journal of Protozoology 26 (3): 444–52. August 1979. doi:10.1111/j.1550-7408.1979.tb04651.x. PMID 536933.
- ↑ "Significance of transovarial infections of Amblyospora sp. (Microspora:Thelohaniidae) in relation to parasite maintenance in the mosquito Culex salinarius". Journal of Invertebrate Pathology 34 (2): 152–7. September 1979. doi:10.1016/0022-2011(79)90095-8. PMID 536610.
- ↑ "A comparison of the life cycles of two Amblyospora (Microspora: Amblyosporidae) in the mosquitoes Culex salinarius and Culex tarsalis Coquillett". Journal of the Florida Anti-Mosquito Association 57 (1): 24–27. 1986.
- ↑ "Amblyospora salinaria n. sp. (Microsporidia: amblyosporidae), parasite of Culex salinarius (Diptera: culicidae): its life cycle stages in an intermediate host". Journal of Invertebrate Pathology 71 (3): 258–62. May 1998. doi:10.1006/jipa.1998.4729. PMID 9538031. https://zenodo.org/record/1229866.
- ↑ Koella, Jacob C.; Lorenz, Lena; Bargielowski, Irka (2009). Chapter 12 Microsporidians as Evolution-Proof Agents of Malaria Control?. Advances in Parasitology. 68. pp. 315–327. doi:10.1016/S0065-308X(08)00612-X. ISBN 978-0-12-374787-7.
- ↑ Baylis, Matthew, ed (2009). "A Possible Mechanism for the Suppression of Plasmodium berghei Development in the Mosquito Anopheles gambiae by the Microsporidian Vavraia culicis". PLOS ONE 4 (3): e4676. doi:10.1371/journal.pone.0004676. PMID 19277119. Bibcode: 2009PLoSO...4.4676B.
- ↑ Herren, JKExpression error: Unrecognized word "etal". (2020). "A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes". Nature Communications 11 (2187): 2187. doi:10.1038/s41467-020-16121-y. PMID 32366903. Bibcode: 2020NatCo..11.2187H.
- ↑ Heinz, E; Williams, TA; Nakjang, S et al. (Oct 2012). "The genome of the obligate intracellular parasite Trachipleistophora hominis: New insights into microsporidian genome dynamics and reductive evolution". PLOS Pathog 8 (10): e1002979. doi:10.1371/journal.ppat.1002979. PMID 23133373.
- ↑ 25.0 25.1 Toguebaye, B. S.; Quilichini, Y.; Diagne, P. M.; Marchand, B. (2014). "Ultrastructure and development of Nosema podocotyloidis n. sp. (Microsporidia), a hyperparasite of Podocotyloides magnatestis (Trematoda), a parasite of Parapristipoma octolineatum (Teleostei)". Parasite 21: 44. doi:10.1051/parasite/2014044. PMID 25174849.

- ↑ Miquel, Jordi; Kacem, Hichem; Baz-González, Edgar; Foronda, Pilar; Marchand, Bernard (2022). "Ultrastructural and molecular study of the microsporidian Toguebayea baccigeri n. gen., n. sp., a hyperparasite of the digenean trematode Bacciger israelensis (Faustulidae), a parasite of Boops boops (Teleostei, Sparidae)". Parasite (EDP Sciences) 29: 2. doi:10.1051/parasite/2022007. ISSN 1776-1042. PMID 35103588.

- ↑ 27.0 27.1 Corradi, N.; Selman, M. (2013). "Latest Progress in Microsporidian Genome Research". Journal of Eukaryotic Microbiology 60 (3): 309–312. doi:10.1111/jeu.12030. PMID 23445243.
- ↑ 28.0 28.1 "The Rad9-Rad1-Hus1 DNA Repair Clamp is Found in Microsporidia". Genome Biology and Evolution 14 (4): evac053. 2022-04-10. doi:10.1093/gbe/evac053. PMID 35439302.
- ↑ Nägeli, C. von (1857). "Über die neue Krankheit der Seidenraupe und verwandte Organismen. pp. 760–61. In: Caspary, R. (ed.). Bericht über die Verhandlungen der 33. Versammlung deutscher Naturforscher und Aerzte, gehalten in Bonn von 18 bis 24 September 1857". Botanische Zeitung 15: 749–776. https://archive.org/stream/botanischezeitun15mohl#page/374/mode/2up.
- ↑ Keeling, P. J.; Fast, N. M. (2002). "Microsporidia: biology and evolution of highly reduced intracellular parasites". Annual Review of Microbiology 56 (1): 93–116. doi:10.1146/annurev.micro.56.012302.160854. PMID 12142484. http://www3.botany.ubc.ca/keeling/PDF/02microARM.pdf.
- ↑ Cavalier-Smith, T (1993). "Kingdom protozoa and its 18 phyla". Microbiological Reviews 57 (4): 953–994. doi:10.1128/MR.57.4.953-994.1993. PMID 8302218.
- ↑ "Simplicity and Complexity of Microsporidian Genomes". Eukaryotic Cell 3 (6): 1363–9. December 2004. doi:10.1128/EC.3.6.1363-1369.2004. PMID 15590811.
- ↑ "Evidence from small-subunit ribosomal RNA sequences for a fungal origin of Microsporidia". Molecular Phylogenetics and Evolution 36 (3): 606–22. September 2005. doi:10.1016/j.ympev.2005.03.031. PMID 15923129.
- ↑ "Loss of the flagellum happened only once in the fungal lineage: phylogenetic structure of Kingdom Fungi inferred from RNA polymerase II subunit genes". BMC Evolutionary Biology 6: 74. 2006. doi:10.1186/1471-2148-6-74. PMID 17010206.
- ↑ "Assessing the microsporidia-fungi relationship: Combined phylogenetic analysis of eight genes". Gene 375: 103–9. June 2006. doi:10.1016/j.gene.2006.02.023. PMID 16626896.
- ↑ "Microsporidia evolved from ancestral sexual fungi". Current Biology 18 (21): 1675–9. November 2008. doi:10.1016/j.cub.2008.09.030. PMID 18976912.
- ↑ "Molecular phylogeny of the Microsporidia: ecological, ultrastructural and taxonomic considerations". Folia Parasitologica 52 (1–2): 131–42; discussion 130. May 2005. doi:10.14411/fp.2005.017. PMID 16004372.
- ↑ Cavalier-Smith (1993). "Kingdom Protozoa and its 18 phyla". Microbiological Reviews 57 (4): 953–94. doi:10.1128/MR.57.4.953-994.1993. PMID 8302218.
- ↑ Alimov, A. F., ed (May 2007). Protista 2: Handbook on zoology. Nauka. pp. 1141. ISBN 9785020262249. https://books.google.com/books?id=FVFQAAAAYAAJ.
- ↑ "Outline of Fungi and fungus-like taxa". Mycosphere 11 (1): 1060–1456. 2020. doi:10.5943/mycosphere/11/1/8. ISSN 2077-7019. http://www.mycosphere.org/pdf/MYCOSPHERE_11_1_8-1.pdf.
External links
- BioHealthBase Bioinformatics Resource Center Database of microspordia sequences and related information.
- Microsporidia at the US National Library of Medicine Medical Subject Headings (MeSH)
Wikidata ☰ Q132652 entry
 |

