Biology:Mycoplasma genitalium
| Mycoplasma genitalium | |
|---|---|
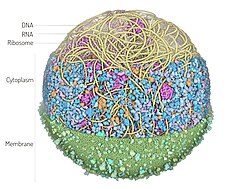
| |
| 3D Whole Cell (3D-WC) model of a Mycoplasma genitalium cell | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Mycoplasmatota |
| Class: | Mollicutes |
| Order: | Mycoplasmoidales |
| Family: | Mycoplasmoidaceae |
| Genus: | Mycoplasmoides |
| Species: | M. genitalium
|
| Binomial name | |
| Mycoplasmoides genitalium (Tully et al. 1983) Gupta et al. 2018[1]
| |
| Synonyms | |
|
Mycoplasma genitalium Tully et al. 1983[2] | |
Mycoplasma genitalium (also known as MG[3], Mgen, or since 2018, Mycoplasmoides genitalium[1]) is a sexually transmitted,[4] small and pathogenic bacterium that lives on the mucous epithelial cells of the urinary and genital tracts in humans.[5] Medical reports published in 2007 and 2015 state that Mgen is becoming increasingly common.[6][7] Resistance to multiple antibiotics, including the macrolide azithromycin, which until recently was the most reliable treatment, is becoming prevalent.[6][8] The bacteria was first isolated from the urogenital tract of humans in 1981,[9] and was eventually identified as a new species of Mycoplasma in 1983.[2] It can cause negative health effects in men and women. It also increases the risk factor for HIV spread[10] with higher occurrences in those previously treated with the azithromycin antibiotics.[6][11]
Symptoms of infection
Mgen is a bacterium recognized for causing urethritis in both men and women along with cervicitis and pelvic inflammation in women.[12] It presents clinically similar symptoms to that of Chlamydia trachomatis infection and has shown higher incidence rates, compared to both Chlamydia trachomatis and Neisseria gonorrhoeae infections in some populations.[13]
Infection with Mgen can be symptomatic or asymptomatic. Both men and women may experience inflammation in the urethra (urethritis), characterized by mucopurulent discharge in the urinary tract, and burning while urinating. In women, it causes cervicitis and pelvic inflammatory diseases (PID), including endometritis and salpingitis.[12] Women may also experience bleeding after sex and it is also linked with tubal factor infertility.[5][14][15] For men, the most common signs are painful urination or a watery discharge from the penis.[16]
There is a consistent association of M. genitalium infection and female reproductive tract syndromes. M. genitalium infection was significantly associated with increased risk of preterm birth, spontaneous abortion, cervicitis, and pelvic inflammatory disease. In addition, this pathogen may latently infect the chorionic villi tissues of pregnant women, thereby impacting pregnancy outcome.[17] Infertility risk is also strongly associated with infection with M. genitalium, although evidence suggests it is not associated with male infertility.[18] When M. genitalium is a co-infectious agent risk associations are stronger and statistically significant.[19]
Polymerase chain reaction analyses indicated that it is a cause of acute non-gonococcal urethritis (NGU) and probably chronic NGU. It is strongly associated with persistent and recurring non-gonococcal urethritis (NGU) responsible for 15 percent to 20 percent of symptomatic NGU cases in men.[20] Unlike other mycoplasmas, the infection is not associated with bacterial vaginosis.[21] It is highly associated with the intensity of HIV infection.[22] Some scientists are performing research to determine if Mgen could play a role in the development of prostate and ovarian cancers and lymphomas in some individuals. These studies have yet to find conclusive evidence to suggest a link.[23]
Genome


The genome of M. genitalium strain G37T consists in one circular DNA of 580,070 base pairs.[24] Scott N. Peterson and his team at the University of North Carolina at Chapel Hill reported the first genetic map using pulsed-field gel electrophoresis in 1991.[25] They performed an initial study of the genome using random sequencing in 1993, by which they found 100,993 nucleotides and 390 protein-coding genes.[26] Collaborating with researchers at The Institute for Genomic Research (TIGR; now the J. Craig Venter Institute), which included Craig Venter, they made the complete genome sequence in 1995 using shotgun sequencing.[24] Only 470 predicted coding regions were identified in 1995, including genes required for DNA replication, transcription and translation, DNA repair, cellular transport, and energy metabolism. It was the second complete bacterial genome ever sequenced, after Haemophilus influenzae.[24] Later data from KEGG reports 476 protein-coding genes and 43 RNA genes, totaling 519.[27] It is unclear where the "525" gene count for the G37T stems from and what gene calling procedure was used.[28]
In 2006, the team at the J. Craig Venter Institute reported that only 382 genes are essential for biological functions.[29] The small genome of M. genitalium made it the organism of choice in The Minimal Genome Project, a study to find the smallest set of genetic material necessary to sustain life.[30]
There is limited divergence among clinical strains of M. genitalium. All strains retain the small genome size.[31]
Diagnosis
Recent research shows that prevalence of Mgen is currently higher than other commonly occurring sexually transmitted infections (STIs).[32] Mgen is a fastidious organism with prolonged growth durations. This makes detection of the pathogen in clinical specimens and subsequent isolation extremely difficult.[33] Lacking a cell wall, Mycoplasma remains unaffected by commonly used antibiotics.[34] The absence of specific serological assays leaves nucleic acid amplification tests (NAAT) as the only viable option for detection of Mgen DNA or RNA.[35] However, samples with positive NAAT for the pathogen should be tested for macrolide resistance mutations, which are strongly correlated to azithromycin treatment failures, owing to rapid rates of mutation of the pathogen.[6] Mutations in the 23S rRNA gene of Mgen have been linked with clinical treatment failure and high level in vitro macrolide resistance.[36] Macrolide resistance mediating mutations have been observed in 20-50% of cases in the UK, Denmark, Sweden, Australia, and Japan.[6] Resistance is also developing towards the second-line antimicrobials like fluoroquinolone.[37]
According to the European guidelines, the indication for commencement of diagnosis for Mgen infection are:[35]
- Detection of nucleic acid (DNA and/or RNA) specific for Mgen in a clinical specimen
- Current partners of individuals who tested positive for Mgen should be treated with the same antimicrobial as the index patient
- If current partner does not attend for evaluation and testing, treatment with the same regimen as given to the index patient should be offered on epidemiological grounds
- On epidemiological grounds for sexual contacts in the previous 3 months; ideally, specimens for a Mgen NAAT should be collected before treatment and treatment should not be given before the result are available
Screening for Mgen with a combination of detection and macrolide resistance mutations will provide the adequate information required to develop personalised antimicrobial treatments, in order to optimise patient management and control the spread of antimicrobial resistance (AMR).[35][38]
Detection of resistance
Owing to the widespread macrolide resistance, samples that are positive for Mgen should ideally be followed up with an assay capable of detecting mutations that mediate antimicrobial resistance. The European Guideline on Mgen infections, in 2016,[39] recommended complementing the molecular detection of Mgen with an assay capable of detecting macrolide resistance-associated mutations.[citation needed]
Treatment
The U.S. Centers for Disease Control and Prevention recommends a step-wise treatment approach for Mycoplasma genitalium with doxycycline for 7 days followed immediately by a 7-day course of moxifloxacin as the preferred therapy due to high rates of macrolide resistance.[40][41] If resistance assay testing is available, and the Mgen is sensitive to macrolides, the CDC recommends a 7-day course of doxycycline followed by a 4-day course of azithromycin. Although the majority of M. genitalium strains are sensitive to moxifloxacin, resistance has been reported, and potential for serious, adverse side effects should be considered with this regimen. [42] Floroquinolones, including Moxifloxacin, have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together including:[citation needed]
- Tendinitis and tendon rupture
- Peripheral Neuropathy
- Central nervous system effects
and other serious side effects detailed in the FDA black box warning. Moxifloxacin/Avelox should be reserved for use when patients have no other treatment options. [43]
In settings without access to resistance testing, or if Moxifloxacin cannot be used, the CDC recommends as an alternative regimen: 7 days of doxycycline followed by the 4-day course of azithromycin, with a test of cure 21 days after treatment being required due to the high rate of macrolide resistance. Beta lactam antibiotics are not effective against Mgen as the organism lacks a cell wall.[41]
In the UK the British Association for Sexual Health and HIV (BASHH) guidelines for treatment are:[44]
- Doxycycline 100mg twice a day for seven days followed by azithromycin 1 gram orally as a single dose then 500mg orally once daily for 2 days where organism is known to be macrolide-sensitive or where resistance status is unknown.
- Moxifloxacin 400mg orally once daily for 10 days if organism known to be macrolide-resistant or where treatment with azithromycin has failed.
Treatment of Mycoplasma genitalium infections is becoming increasingly difficult due to rapidly growing antimicrobial resistance.[45] Diagnosis and treatment is further hampered by the fact that Mycoplasma genitalium infections are not routinely tested.[46] Studies have demonstrated that a 5-day course of azithromycin has a superior cure rate compared to a single, larger dose. Further, a single dose of azithromycin can lead to the bacteria becoming resistant to azithromycin.[47] Among Swedish patients, doxycycline was shown to be relatively ineffective (with a cure rate of 48% for women and 38% for men); and treatment with a single dose of azithromycin is not prescribed due to it inducing antimicrobial resistance. The five-day treatment with azithromycin showed no development of antimicrobial resistance.[48] Based on these findings, UK doctors are moving to the 5-day azithromycin regimen. Doxycycline is also still used, and moxifloxacin is used as a second-line treatment in case doxycyline and azithromycin are not able to eradicate the infection.[49][50] In patients where doxycycline, azithromycin and moxifloxacin all failed, pristinamycin has been shown to still be able to eradicate the infection.[49]
History
Mycoplasma genitalium was originally isolated in 1980 from urethral specimens of two male patients with non-gonococcal urethritis in the genitourinary medicine (GUM) clinic at St Mary's Hospital, Paddington, London.[51][52] It was reported in 1981 by a team led by Joseph G. Tully.[9] Under electron microscopy, it appears as a flask-shaped cell with a narrow terminal portion that is crucial for its attachment to the host cell surfaces.[53] The bacterial cell is slightly elongated somewhat like a vase, and measures 0.6–0.7 μm in length, 0.3–0.4 μm at the broadest region, and 0.06–0.08 μm at the tip. The base is broad while the tip is stretched into a narrow neck, which terminates with a cap. The terminal region has a specialised region called nap, which is absent in other mycoplasmas. Serological tests indicated that the bacterium was not related to known species of Mycoplasma. The comparison of genome sequences with other urinogenital bacteria, such as M. hominis and Ureaplasma parvum, revealed that M. genitalium is significantly different, especially in the energy-generating pathways, although it shared a core genome of ~250 protein-encoding genes.[54]
In 2018, Gupta et al. proposed to change the name of Mycoplasma genitalium to Mycoplasmoides genitalium on phylogenetic grounds, reflecting the existing knowledge that M. genitalium is not very related to other Mycoplasma.[1] The change became correct name under the International Code of Nomenclature of Prokaryotes (ICNP, "Code") with Validation List 184, published by the ICSP ("Committee").[55] Mycoplasmaologists working in the field generally oppose this renaming. In 2019, they published an opinion paper arguing that even though the phylogenetic methods are valid, Gupta's renaming scheme causes too many changes, which is impractical and confusing.[56] They cite some essential principles of the Code, such as "no unnecessary new names", "aim at stability of names", and "avoid or reject the use of names which may cause error or confusion".[56] However, the 2019 argument for preserving old names was rejected by the Committee in Opinion 122 of 2022,[57] where it was ruled that the argument incorrectly cited the Code.[55] The Opinion emphasizes that use of an older validly published name remains acceptable under the Code.[57]
Synthetic genome
On 6 October 2007, Craig Venter announced that a team of scientists led by Nobel laureate Hamilton Smith at the J. Craig Venter Institute had successfully constructed synthetic DNA with which they planned to make the first synthetic genome. Reporting in The Guardian , Venter said that they had stitched together a DNA strand containing 381 genes, consisting of 580,000 base pairs, based on the genome of M. genitalium.[58] On 24 January 2008, they announced the successful creation of a synthetic bacterium, which they named Mycoplasma genitalium JCVI-1.0 (the name of the strain indicating J. Craig Venter Institute with its specimen number).[59] They synthesised and assembled the complete 582,970-base pair genome of the bacterium. The final stages of synthesis involved cloning the DNA into the bacterium E. coli for nucleotide production and sequencing. This produced large fragments of approximately 144,000 base pairs or 1/4th of the whole genome. Finally, the products were cloned inside the yeast Saccharomyces cerevisiae to synthesize the 580,000 base pairs.[60][61] The molecular size of the synthetic bacterial genome is 360,110 kilodaltons (kDa). Printed in 10-point font, the letters of the genome cover 147 pages.[62]
On 20 July 2012, Stanford University and the J. Craig Venter Institute announced successful simulation of the complete life cycle of a Mycoplasma genitalium cell, in the journal Cell.[63] The entire organism is modeled in terms of its molecular components, integrating all cellular processes into a single model. Using object oriented programming to model the interactions of 28 categories of molecules, including DNA, RNA, proteins, and metabolites, and running on a 128 computer Linux cluster, the simulation takes 10 hours for a single M. genitalium cell to divide once—about the same time the actual cell takes—and generates half a gigabyte of data.[64]
Research
The discovery of Protein M, a new protein from M. genitalium, was announced in February 2014.[65] The protein was identified during investigations on the origin of multiple myeloma, a B-cell hematologic neoplasm. To understand the long-term Mycoplasma infection, it was found that antibodies from multiple myeloma patients' blood were recognised by M. genitalium. The antibody reactivity was due to a protein never known before, and is chemically responsive to all types of human and nonhuman antibodies available. The protein is about 50 kDa in size, and composed of 556 amino acids.[66] Mgen evolved from a gram-positive ancestor that was clostridium-like but has lost the genes to code for the enzymes involved in de novo nucleic acid synthesis, amino acid synthesis, and synthesis of fatty acids.[67] This means that Mgen needs the host's growth factors to keep reproducing. Although Mgen has abilities that help it adhere to cells, it is still unknown how the bacteria can maintain an infection inside the epithelial cells of the ectocervix and vagina when shedding of the apical layer of cells occur. The organism's ability to have adhesion to host cells relies of two proteins, P110 and P140. Adhesion is an important step in beginning an infection in a cell and Mgen can adhere to spermatozoa, erythrocytes, and epithelial cells. The terminal organelle relies on these proteins as well because without them the organelle was not present. The segmented pair plates of Mgen [vague] is a core of dense electrons which is anchored to the cell membrane. The end of this core is in contact with the wheel complex and the wheel complex contains the proteins MG219, MG200, MG386, and MG491 which aid in the gliding motility of the bacteria. Although Mgen lacks secreted virulence factors, the protein MG186 degrades host nucleic acids due to it being a calcium-dependent membrane-associated nuclease.[citation needed]
See also
- Smallest organisms
References
- ↑ 1.0 1.1 1.2 Gupta, Radhey S.; Sawnani, Sahil; Adeolu, Mobolaji; Alnajar, Seema; Oren, Aharon (2018-09-01). "Phylogenetic framework for the phylum Tenericutes based on genome sequence data: proposal for the creation of a new order Mycoplasmoidales ord. nov., containing two new families Mycoplasmoidaceae fam. nov. and Metamycoplasmataceae fam. nov. harbouring Eperythrozoon, Ureaplasma and five novel genera" (in en). Antonie van Leeuwenhoek 111 (9): 1583–1630. doi:10.1007/s10482-018-1047-3. ISSN 1572-9699. PMID 29556819. https://doi.org/10.1007/s10482-018-1047-3.
- ↑ 2.0 2.1 Tully, J. G.; Taylor-Robinson, D.; Rose, D. L.; Cole, R. M.; Bove, J. M. (1983). "Mycoplasma genitalium, a New Species from the Human Urogenital Tract". International Journal of Systematic Bacteriology 33 (2): 387–396. doi:10.1099/00207713-33-2-387.
- ↑ Roberts, Michelle (11 July 2018). "Emerging sex disease 'could be superbug'". BBC News. https://www.bbc.com/news/health-44777938.
- ↑ Workowski K. A., Bolan G. A. (2015). "Sexually transmitted diseases treatment guidelines, 2015". MMWR Recomm. Rep. 64 (RR-03): 1–137. PMID 26042815.
- ↑ 5.0 5.1 Weinstein, Scott A.; Stiles, Bradley G. (2012-01-01). "Recent perspectives in the diagnosis and evidence-based treatment of Mycoplasma genitalium". Expert Review of Anti-infective Therapy 10 (4): 487–499. doi:10.1586/eri.12.20. ISSN 1478-7210. PMID 22512757.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Management of Mycoplasma genitalium infections—Can we hit a moving target". BMC Infect Dis 15: 343. 2015. doi:10.1186/s12879-015-1041-6. PMID 26286546.
- ↑ "Mycoplasma genitalium among young adults in the United States: An emerging sexually transmitted infection". Am J Public Health 97 (6): 1118–1125. 2007. doi:10.2105/ajph.2005.074062. PMID 17463380.
- ↑ Patel, Parth H.; Hashmi, Muhammad F. (2023), "Macrolides", StatPearls (Treasure Island (FL): StatPearls Publishing), PMID 31855339, http://www.ncbi.nlm.nih.gov/books/NBK551495/, retrieved 2023-09-25
- ↑ 9.0 9.1 Tully, Joseph G.; Cole, Roger M.; Taylor-Robinson, David; Rose, David L. (1981). "A newly discovered Mycoplasma in the human urinogenital tract". The Lancet 317 (8233): 1288–1291. doi:10.1016/S0140-6736(81)92461-2. PMID 6112607.
- ↑ World Health Organization (WHO). Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus. Switzerland: World Health Organization 2013
- ↑ Barberá M, et al. Mycoplasma genitalium macrolide and fluoroquino-lone resistance: prevalence and risk factors among a 2013–2014 cohort of patients in Barcelona Sexually Transmitted Diseases: Spain, 2017; In Press
- ↑ 12.0 12.1 Wiesenfeld, Harold C.; Manhart, Lisa E. (2017-07-15). "Mycoplasma genitalium in Women: Current Knowledge and Research Priorities for This Recently Emerged Pathogen". The Journal of Infectious Diseases 216 (suppl_2): S389–S395. doi:10.1093/infdis/jix198. ISSN 1537-6613. PMID 28838078.
- ↑ "Prevalence of chlamydial and gonococcal infections among young adults in the United States". JAMA 291 (18): 2229–2236. 2004. doi:10.1001/jama.291.18.2229. PMID 15138245.
- ↑ Manhart, Lisa E. (2013). "Mycoplasma genitalium: An emergent sexually transmitted disease?". Infectious Disease Clinics of North America 27 (4): 779–792. doi:10.1016/j.idc.2013.08.003. PMID 24275270.
- ↑ Schorge, John O.; Halvorson, Lisa M.; Schaffer, Joseph I.; Corton, Marlene M.; Bradshaw, Karen D.; Hoffman, Barbara L. (2016-04-22). Williams gynecology. Schorge, John O.,, Hoffman, Barbara L.,, Bradshaw, Karen D.,, Halvorson, Lisa M.,, Schaffer, Joseph I.,, Corton, Marlene M. (Third ed.). New York. pp. 65. ISBN 9780071849081. OCLC 944920918.
- ↑ Health.com, Kristine Thomason (7 December 2015). "What you should know about this 'new' STD - CNN". http://www.cnn.com/2015/12/07/health/what-to-know-std-mg/index.html.
- ↑ "Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage.". J Cell Physiol 234 (1): 100–9107. 2018. doi:10.1002/jcp.26952. PMID 30078192.
- ↑ C. Huang; H.L. Zhu; K.R. Xu; S.Y. Wang; L.Q. Fan; W.B. Zhu (September 2015). "Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis". Andrology 3 (5): 809–816. doi:10.1111/andr.12078. PMID 26311339.
- ↑ Lis, R.; Rowhani-Rahbar, A.; Manhart, L. E. (2015). "Mycoplasma genitalium Infection and Female Reproductive Tract Disease: A Meta-Analysis". Clinical Infectious Diseases 61 (3): 418–426. doi:10.1093/cid/civ312. ISSN 1058-4838. PMID 25900174.
- ↑ Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin InfeAIDSct Dis. 2015;61(3):418-426
- ↑ Taylor-Robinson, D. (2002). "Mycoplasma genitalium – an up-date". International Journal of STD & AIDS 13 (3): 145–151. doi:10.1258/0956462021924776. PMID 11860689.
- ↑ Weinstein, Scott A; Stiles, Bradley G (2013). "Recent perspectives in the diagnosis and evidence-based treatment of". Expert Review of Anti-infective Therapy 10 (4): 487–499. doi:10.1586/eri.12.20. PMID 22512757.
- ↑ Zarei, Omid; Rezania, Simin; Mousavi, Atefeh (2013). "Mycoplasma genitalium and Cancer: A Brief Review". Asian Pacific Journal of Cancer Prevention 14 (6): 3425–3428. doi:10.7314/APJCP.2013.14.6.3425. ISSN 1513-7368. PMID 23886122.
- ↑ 24.0 24.1 24.2 Fraser, C. M.; Gocayne, J. D.; White, O.; Adams, M. D.; Clayton, R. A.; Fleischmann, R. D.; Bult, C. J.; Kerlavage, A. R. et al. (1995). "The Minimal Gene Complement of Mycoplasma genitalium". Science 270 (5235): 397–404. doi:10.1126/science.270.5235.397. PMID 7569993. Bibcode: 1995Sci...270..397F.
- ↑ Peterson, Scott N.; Schramm, Nara; Hu, Ping-chuan; Bott, Kenneth F.; Hutchison, Clyde A. (1991). "A random sequencing approach for placing markers on the physical map of". Nucleic Acids Research 19 (21): 6027–6031. doi:10.1093/nar/19.21.6027. PMID 1945886.
- ↑ Peterson, SN; Hu, PC; Bott, KF; Hutchison CA, 3rd (1993). "A survey of the Mycoplasma genitalium genome by using random sequencing". Journal of Bacteriology 175 (24): 7918–7930. doi:10.1128/jb.175.24.7918-7930.1993. PMID 8253680.
- ↑ "KEGG GENOME: Mycoplasmoides genitalium G37". https://www.genome.jp/entry/gn:T00002.
- ↑ Karr, JR; Sanghvi, JC; Macklin, DN; Gutschow, MV; Jacobs, JM; Bolival B, Jr; Assad-Garcia, N; Glass, JI et al. (20 July 2012). "A whole-cell computational model predicts phenotype from genotype.". Cell 150 (2): 389–401. doi:10.1016/j.cell.2012.05.044. PMID 22817898.
- ↑ Glass, J. I.; Assad-Garcia, N.; Alperovich, N.; Yooseph, S.; Lewis, M. R.; Maruf, M.; Hutchison, C. A.; Smith, H. O. et al. (2006). "Essential genes of a minimal bacterium". Proceedings of the National Academy of Sciences 103 (2): 425–430. doi:10.1073/pnas.0510013103. PMID 16407165. Bibcode: 2006PNAS..103..425G.
- ↑ Razin, S (1997). "The minimal cellular genome of mycoplasma". Indian Journal of Biochemistry & Biophysics 34 (1–2): 124–30. PMID 9343940.
- ↑ Fookes, MC; Hadfield, J; Harris, S; Parmar, S; Unemo, M; Jensen, JS; Thomson, NR (28 December 2017). "Mycoplasma genitalium: whole genome sequence analysis, recombination and population structure.". BMC Genomics 18 (1): 993. doi:10.1186/s12864-017-4399-6. PMID 29281972.
- ↑ Taylor-Robinson D, Jensen JS (2011). "Mycoplasma genitalium: from chrysalis to multicolored butterfly". Clin Microbiol Rev 24 (3): 498–514. doi:10.1128/cmr.00006-11. PMID 21734246.
- ↑ Jensen J. S., Hansen H. T., Lind K. (1996). "Isolation of Mycoplasma genitalium strains from the male urethra". J. Clin. Microbiol. 34 (2): 286–291. doi:10.1128/jcm.34.2.286-291.1996. PMID 8789002.
- ↑ Centers for Disease Control and Prevention, 2015 Sexually Transmitted Diseases Treatment Guidelines.
- ↑ 35.0 35.1 35.2 Jensen JS, Cusini M, Gomberg M, Moi H (2016). "2016 European guideline on Mycoplasma genitalium infections.". J Eur Acad Dermatol Venereol 30 (10): 1650–1656. doi:10.1111/jdv.13849. PMID 27505296.
- ↑ "Azithromycin Treatment Failure in Mycoplasma genitalium–Positive Patients with Nongonococcal Urethritis Is Associated with Induced Macrolide Resistance". Clin. Infect. Dis. 47 (12): 1546–1553. 2008. doi:10.1086/593188. PMID 18990060.
- ↑ Unemo, M. & Jensen, J.S. ‘Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium’. 2016. Nat. Rev. Urol..268. Published online 10 January 2017. doi:10.1038/nrurol
- ↑ Tabrizi SN et al. Multiplex Assay for Simultaneous Detection of Mycoplasma genitalium and Macrolide Resistance Using PlexZyme and PlexPrime Technology. PLoS ONE. 2016. 11(6): e0156740. doi:10.1371/journal.pone.0156740
- ↑ "2016 European guideline on Mycoplasma genitalium infections". J Eur Acad Dermatol Venereol 30 (10): 1650–1656. 2016. doi:10.1111/jdv.13849. PMID 27505296.
- ↑ Tuddenham, Susan; Hamill, Matthew M.; Ghanem, Khalil G. (11 January 2022). "Diagnosis and Treatment of Sexually Transmitted Infections: A Review". JAMA 327 (2): 161–172. doi:10.1001/jama.2021.23487. PMID 35015033.
- ↑ 41.0 41.1 Workowski, Kimberly A. (2021). "Sexually Transmitted Infections Treatment Guidelines, 2021". MMWR. Recommendations and Reports 70 (4): 1–187. doi:10.15585/mmwr.rr7004a1. PMID 34292926.
- ↑ "Food and Drug Administration". //www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse.
- ↑ "Access Data FDA". https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021085s063lbl.pdf.
- ↑ "BASHH Guidelines". https://www.bashh.org/guidelines.
- ↑ "Yaws". 2013. https://www.who.int/yaws/2013_Yaws_seminar_Lancet.pdf?ua=1&ua=1.
- ↑ Suneta, Soni; Parkhouse, Andy; Gillian, Dean (24 April 2017). "Macrolide and quinolone-resistant Mycoplasma genitalium in a man with persistent urethritis: the tip of the British iceberg?". Sexually Transmitted Infections 93 (8): sextrans–2016–053077. doi:10.1136/sextrans-2016-053077. PMID 28438948.
- ↑ Yew, H. S.; Anderson, T.; Coughlan, E.; Werno, A. (2011). "Induced macrolide resistance in Mycoplasma genitalium isolates from patients with recurrent nongonococcal urethritis". Journal of Clinical Microbiology 49 (4): 1695–1696. doi:10.1128/JCM.02475-10. PMID 21346049.
- ↑ Anagrius, Carin; Loré, Britta; Jensen, Jørgen Skov; Coenye, Tom (2013). "Treatment of Mycoplasma genitalium. Observations from a Swedish STD Clinic". PLOS ONE 8 (4): e61481. doi:10.1371/journal.pone.0061481. PMID 23593483. Bibcode: 2013PLoSO...861481A.
- ↑ 49.0 49.1 Unemo, Magnus; Jensen, Jorgen S. (10 January 2017). "Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium". Nature Reviews Urology 14 (3): 139–125. doi:10.1038/nrurol.2016.268. PMID 28072403.
- ↑ "Mycoplasma Genitalium Treatment Choices". http://www.theonlineclinic.co.uk/news/2012/12/20/MycoplasmaGenitaliumTreatmentChoices.aspx.
- ↑ Taylor-Robinson, D.; Horner, P. J. (2001). "The role of Mycoplasma genitalium in non-gonococcal urethritis". Sexually Transmitted Infections 77 (4): 229–231. doi:10.1136/sti.77.4.229. PMID 11463919.
- ↑ Daley, G.; Russell, D.; Tabrizi, S.; McBride, J. (2014). "Mycoplasma genitalium: a review". International Journal of STD & AIDS 25 (7): 475–487. doi:10.1177/0956462413515196. PMID 24517928.
- ↑ Taylor-Robinson, D (1995). "The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases". Genitourinary Medicine 71 (1): 1–8. doi:10.1136/sti.71.1.1. PMID 7750946.
- ↑ Blanchard, Alain; Bébéar, Cécile (2011). "The evolution of Mycoplasma genitalium". Annals of the New York Academy of Sciences 1230 (1): E61–E64. doi:10.1111/j.1749-6632.2011.06418.x. PMID 22417108. Bibcode: 2011NYASA1230E..61B.
- ↑ 55.0 55.1 "Genus: Mycoplasmoides" (in en). https://lpsn.dsmz.de/genus/mycoplasmoides.
- ↑ 56.0 56.1 Balish, Mitchell; Bertaccini, Assunta; Blanchard, Alain; Brown, Daniel; Browning, Glenn; Chalker, Victoria; Frey, Joachim; Gasparich, Gail et al. (2019). "Recommended rejection of the names Malacoplasma gen. nov., Mesomycoplasma gen. nov., Metamycoplasma gen. nov., Metamycoplasmataceae fam. nov., Mycoplasmoidaceae fam. nov., Mycoplasmoidales ord. nov., Mycoplasmoides gen. nov., Mycoplasmopsis gen. nov. [Gupta, Sawnani, Adeolu, Alnajar and Oren 2018] and all proposed species comb. nov. placed therein". International Journal of Systematic and Evolutionary Microbiology 69 (11): 3650–3653. doi:10.1099/ijsem.0.003632. ISSN 1466-5034. PMID 31385780.
- ↑ 57.0 57.1 Arahal, David R.; Busse, Hans-Jürgen; Bull, Carolee T.; Christensen, Henrik; Chuvochina, Maria; Dedysh, Svetlana N.; Fournier, Pierre-Edouard; Konstantinidis, Konstantinos T. et al. (10 August 2022). "Judicial Opinions 112–122". International Journal of Systematic and Evolutionary Microbiology 72 (8). doi:10.1099/ijsem.0.005481. PMID 35947640. https://www.researchgate.net/publication/362626093.
- ↑ Pilkington, Ed (6 October 2007). "I am creating artificial life, declares US gene pioneer". The Guardian (Guardian News and Media Limited). https://www.theguardian.com/science/2007/oct/06/genetics.climatechange.
- ↑ Kowalski, Heather. "Venter Institute Scientists Create First Synthetic Bacterial Genome". J. Craig Venter Institute. http://www.jcvi.org/cms/research/projects/synthetic-bacterial-genome/press-release/.
- ↑ Gibson, D. G.; Benders, G. A.; Andrews-Pfannkoch, C.; Denisova, E. A.; Baden-Tillson, H.; Zaveri, J.; Stockwell, T. B.; Brownley, A. et al. (2008). "Complete Chemical Synthesis, Assembly, and Cloning of a Mycoplasma genitalium Genome". Science 319 (5867): 1215–1220. doi:10.1126/science.1151721. PMID 18218864. Bibcode: 2008Sci...319.1215G.
- ↑ Ball, Philip (2008-01-24). "Genome stitched together by hand". Nature News. doi:10.1038/news.2008.522. http://www.nature.com/news/2008/080124/full/news.2008.522.html.
- ↑ "Scientists Create First Synthetic Bacterial Genome -- Largest Chemically Defined Structure Synthesized In The Lab". ScienceDaily. 24 January 2008. https://www.sciencedaily.com/releases/2008/01/080124175924.htm.
- ↑ Karr, Jonathan R.; Sanghvi, Jayodita C.; Macklin, Derek N.; Gutschow, Miriam V.; Jacobs, Jared M.; Bolival, Benjamin; Assad-Garcia, Nacyra; Glass, John I. et al. (2010). "A Whole-Cell Computational Model Predicts Phenotype from Genotype". Cell 150 (2): 389–401. doi:10.1016/j.cell.2012.05.044. PMID 22817898.
- ↑ "In First, Software Emulates Lifespan of Entire Organism". The New York Times. 20 July 2012. https://www.nytimes.com/2012/07/21/science/in-a-first-an-entire-organism-is-simulated-by-software.html.
- ↑ "The Ultimate Decoy: Scripps Research Institute Scientists Find Protein that Helps Bacteria Misdirect Immune System". The Scripps Research Institute (TSRI). http://www.scripps.edu/news/press/2014/20140206lerner.html.
- ↑ Grover, R. K.; Zhu, X.; Nieusma, T.; Jones, T.; Boero, I.; MacLeod, A. S.; Mark, A.; Niessen, S. et al. (2014). "A structurally distinct human mycoplasma protein that generically blocks antigen-antibody union". Science 343 (6171): 656–661. doi:10.1126/science.1246135. PMID 24503852. Bibcode: 2014Sci...343..656G.
- ↑ Raj, Stephen (2022). "Mycoplasma genitalium : A new superbug". Indian Journal of Sexually Transmitted Diseases and AIDS 43 (2589–0557): 1–12. doi:10.4103/ijstd.ijstd_103_20. PMID 35846530.
External links
- Mycoplasma genitalium Reference Work at the UK Health Protection Agency
- Type strain of Mycoplasma genitalium at BacDive - the Bacterial Diversity Metadatabase
Wikidata ☰ Q1930909 entry
 |
